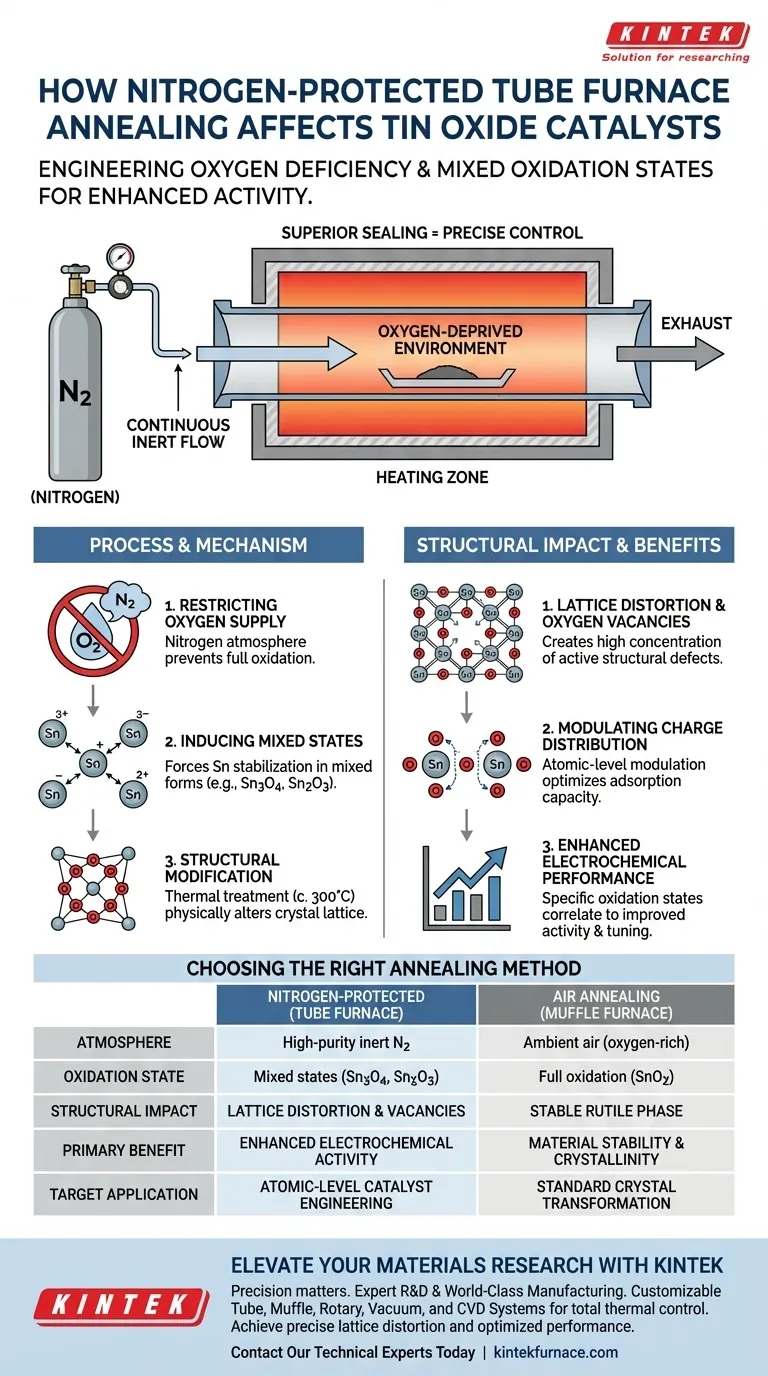
Nitrogen-protected annealing in a tube furnace fundamentally alters the microscopic structure of tin oxide by forcing the material into an oxygen-deficient state. Because the furnace’s superior sealing capabilities allow for a continuous flow of inert nitrogen, the process restricts oxygen availability, preventing full oxidation and inducing the formation of mixed oxidation states such as Sn3O4 or Sn2O3.
The tube furnace creates a precise, oxygen-deprived thermal environment that modifies the catalyst's charge distribution through lattice distortion and oxygen vacancies, significantly influencing its electrochemical performance.

Controlling the Oxidation Environment
The Function of Oxygen Deficiency
The defining characteristic of this process is the restriction of oxygen supply. Unlike treatment in an air atmosphere, which typically results in fully oxidized forms, the nitrogen environment creates a deficit.
This deficit forces the tin oxide to stabilize in mixed oxidation states. Instead of forming pure SnO2, the material develops intermediate structures like Sn3O4 or Sn2O3.
Sealing and Atmosphere Control
The efficacy of this structural change relies on the superior sealing capabilities of the high-temperature tube furnace.
This ensures a pure inert atmosphere is maintained throughout the heating cycle. Even a small leak of oxygen could revert the material to a standard rutile phase, negating the benefits of the annealing process.
Mechanism of Structural Modification
Inducing Lattice Distortion
The thermal treatment, often conducted around 300 degrees Celsius, does more than just heat the material; it physically alters the crystal lattice.
The oxygen-deficient environment promotes lattice distortion. This physical warping of the atomic structure creates a high concentration of oxygen vacancies.
Modulating Charge Distribution
These structural defects and vacancies are not flaws; they are active features. They cause an atomic-level modulation of charge distribution.
This redistribution changes how the catalyst interacts with other chemicals. It optimizes the adsorption capacity of active sites, making the catalyst more effective at binding reactant molecules.
Enhancing Electrochemical Performance
The ultimate result of these microscopic changes is a shift in performance.
By controlling the initial oxidation state through nitrogen annealing, researchers can tune the catalyst. This allows for precise studies on how specific oxidation states directly correlate to improved electrochemical activity.
Understanding the Trade-offs
Stability vs. Activity
While nitrogen annealing enhances activity through vacancies, it produces a material that is thermodynamically less stable than fully oxidized tin oxide.
Standard annealing in a box muffle furnace under air (typically at higher temperatures like 370°C–525°C) produces stable, tetragonal rutile phase SnO2.
Complexity of Control
Achieving specific mixed states (like Sn3O4) requires rigorous control over gas flow and seal integrity.
If the goal is simply to regulate grain size or transform amorphous precursors into standard crystals, the nitrogen-protected tube furnace adds unnecessary complexity compared to standard air annealing.
Making the Right Choice for Your Goal
To select the correct annealing method, you must define the specific structural properties required for your catalyst.
- If your primary focus is optimizing electrochemical activity: Use a tube furnace with nitrogen flow to induce oxygen vacancies, lattice distortion, and mixed oxidation states.
- If your primary focus is material stability and crystallinity: Use a box muffle furnace in air to produce fully oxidized, stable tetragonal rutile phase SnO2.
By manipulating the annealing atmosphere, you move beyond simple heating to precise atomic-level engineering of the catalyst.
Summary Table:
| Feature | Nitrogen-Protected (Tube Furnace) | Air Annealing (Muffle Furnace) |
|---|---|---|
| Atmosphere Control | High-purity inert nitrogen flow | Ambient air (oxygen-rich) |
| Oxidation State | Mixed states (Sn3O4, Sn2O3) | Full oxidation (SnO2) |
| Structural Impact | Lattice distortion & oxygen vacancies | Stable tetragonal rutile phase |
| Primary Benefit | Enhanced electrochemical activity | Material stability & crystallinity |
| Target Application | Atomic-level catalyst engineering | Standard crystal transformation |
Elevate Your Materials Research with KINTEK
Precision matters when engineering the next generation of catalysts. Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems designed to give you total control over your thermal environment.
Whether you need superior sealing for nitrogen-protected annealing or robust systems for high-temperature oxidation, our lab furnaces are fully customizable to meet your unique research requirements. Don't settle for inconsistent results—partner with KINTEK to achieve precise lattice distortion and optimized electrochemical performance.
Contact Our Technical Experts Today to find the perfect furnace solution for your laboratory.
Visual Guide
References
- Nicolò B. D. Monti, Katarzyna Bejtka. Effects of Annealing Conditions on the Catalytic Performance of Anodized Tin Oxide for Electrochemical Carbon Dioxide Reduction. DOI: 10.3390/nano15020121
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What are the limitations of stainless steel tube furnaces? Overcome Temperature and Contamination Issues
- How do the heating elements in a tube furnace function? Uncover Key Insights for Precise Heating
- How does a tube furnace improve the crystal structure of zinc oxide thin films? Achieve High-Performance Crystallinity
- Why is a programmable tube furnace required for the synthesis of bulk Cu13Se52Bi35 alloys? Essential Thermal Precision
- In which industries and research domains are vertical tube furnaces commonly used? Essential for Precision Thermal Processing
- What factors determine the selection of a three-zone split tube furnace? Key Specs for Precision Thermal Processing
- What technical features make a laboratory horizontal tube furnace an ideal reaction device for oil sludge studies?
- What heat treatment processes can a 70mm tube furnace be used for? Essential Guide for Material Processing



















