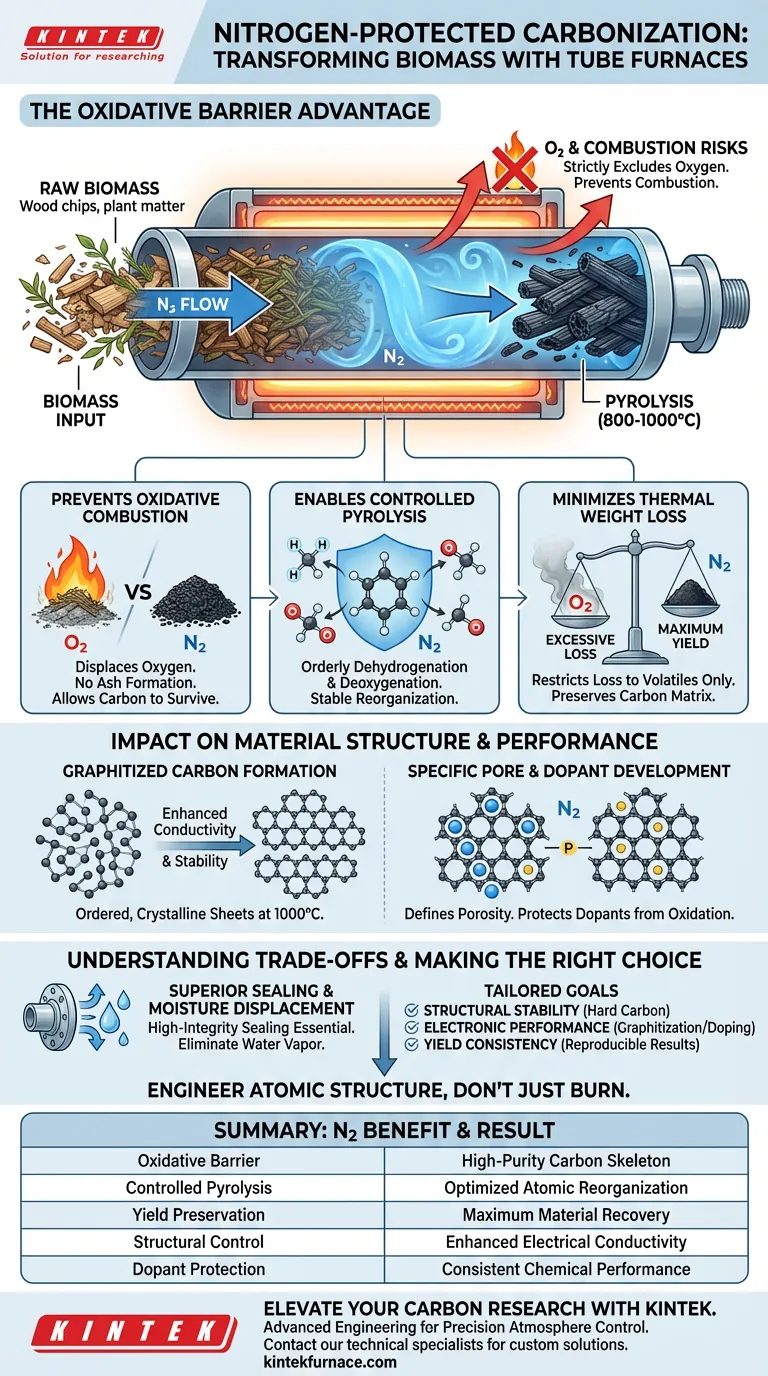
A nitrogen-protected environment primarily acts as an oxidative barrier, strictly excluding oxygen from the reaction chamber during high-temperature processing. By continuously displacing oxygen, the tube furnace ensures that biomass undergoes pyrolysis (thermal decomposition) rather than combustion (burning), allowing the material to convert into stable carbon at temperatures ranging from 800 °C to 1000 °C.
Core Takeaway The nitrogen atmosphere shifts the chemical process from destructive burning to constructive restructuring. It allows the biomass to shed volatile components and reorganize its atomic structure into high-quality, graphitized carbon without losing mass to oxidative reactions.
The Critical Role of Inert Atmospheres
Prevention of Oxidative Combustion
At temperatures exceeding 800 °C, biomass is highly reactive. Without a protective atmosphere, the presence of oxygen would cause the carbon precursor to ignite and burn away, leaving only ash. The continuous flow of nitrogen displaces oxygen, creating the inert conditions necessary for carbon atoms to survive the thermal shock.
Enabling Controlled Pyrolysis
Carbonization requires the orderly removal of non-carbon elements like hydrogen and oxygen. The nitrogen environment facilitates this "orderly dehydrogenation and deoxygenation." This creates a stable reductive or neutral atmosphere where the carbon skeleton can reorganize itself without interference from external oxidizers.
Minimizing Thermal Weight Loss
Uncontrolled oxidation leads to excessive mass loss, reducing the final yield of the carbon material. By strictly limiting oxygen exposure, the nitrogen atmosphere ensures that weight loss is restricted to the release of volatile components only. This preserves the structural integrity of the remaining carbon matrix.
Impact on Material Structure and Performance
Formation of Graphitized Carbon
The primary reference highlights that a nitrogen-protected environment is essential for producing graphitized carbon materials. The absence of oxygen allows carbon atoms to align into ordered, crystalline sheets (graphitization) during heating at 1000 °C, which significantly enhances the material's electrical conductivity and chemical stability.
Development of Specific Pore Structures
A controlled atmosphere is vital for defining the porosity of the final product. By preventing the collapse of the carbon framework due to oxidation, the furnace allows for the creation of specific pore structures. These pores are critical for applications requiring high surface area, such as in battery electrodes or filtration systems.
Facilitating Precise Doping
When introducing foreign atoms like phosphorus to enhance performance, the nitrogen atmosphere protects both the carbon and the dopant. It prevents the "dopant source" from oxidizing before it can integrate into the carbon lattice. This ensures high consistency and stability in the resulting doped materials.
Understanding the Trade-offs
The Necessity of Superior Sealing
A continuous nitrogen flow is ineffective if the tube furnace lacks high-integrity sealing. Even minor leaks can introduce enough ambient oxygen to trigger "undesirable oxidation side reactions," compromising the purity of the inert atmosphere.
Moisture Displacement
Nitrogen does not just displace oxygen; it must also displace moisture. Residual moisture can act as an oxidizing agent at high temperatures. Effective processing often requires segmented heating programs (e.g., holding at 100 °C) under nitrogen flow to fully eliminate water vapor before higher temperatures are reached.
Making the Right Choice for Your Goal
- If your primary focus is Structural Stability: Ensure your furnace creates a strictly inert environment to prevent oxidative loss, allowing for the formation of hard carbon with distinct interlayer spacing.
- If your primary focus is Electronic Performance: Prioritize a high-purity nitrogen flow to facilitate graphitization and protect dopants (like phosphorus) from oxidation during the heating curve.
- If your primary focus is Yield Consistency: Use a furnace with precise sealing and flow control to minimize unnecessary thermal weight loss and ensure reproducible carbonization degrees.
Ultimately, the nitrogen environment is the fundamental control variable that allows you to engineer the atomic structure of carbon rather than simply burning biomass.
Summary Table:
| Benefit Feature | Impact on Carbonization Process | Resulting Material Advantage |
|---|---|---|
| Oxidative Barrier | Displaces oxygen to prevent combustion and ash formation | High-purity carbon skeleton |
| Controlled Pyrolysis | Enables orderly removal of hydrogen and oxygen | Optimized atomic reorganization |
| Yield Preservation | Restricts mass loss to volatile components only | Maximum material recovery and density |
| Structural Control | Facilitates graphitization at 1000 °C | Enhanced electrical conductivity |
| Dopant Protection | Prevents oxidation of additives (e.g., Phosphorus) | Consistent chemical performance |
Elevate Your Carbon Research with KINTEK
Precision in atmosphere control is the difference between high-performance carbon and simple ash. At KINTEK, we understand that your biomass research requires rigorous oxygen exclusion and stable thermal environments.
Why partner with us?
- Advanced Engineering: Our Tube, Muffle, and Vacuum furnaces feature high-integrity sealing systems to ensure a perfectly inert nitrogen environment.
- Customizable Solutions: Backed by expert R&D and manufacturing, we offer CVD systems and high-temp furnaces tailored to your specific biomass carbonization or doping needs.
- Industry Expertise: We provide the tools necessary for precise pore-structure development and consistent yield reproduction.
Ready to optimize your pyrolysis results? Contact our technical specialists today to find the perfect furnace for your laboratory’s unique requirements.
Visual Guide
References
- Feng Yang, Wei Sun. A Portable Electrochemical Dopamine Detector Using a Fish Scale-Derived Graphitized Carbon-Modified Screen-Printed Carbon Electrode. DOI: 10.3390/molecules29030744
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why is a water-cooling spray system implemented in annealing? Maximize Production Throughput & Material Quality
- What are inert atmosphere conditions? Achieve Purity and Control in Your Processes
- Why Use a Nitrogen Debinding Furnace for 17-4PH? Ensure Pure Metal Bonds & Prevent Oxidation
- What are the main advantages of using a retort furnace? Achieve Superior Control and Purity in Heat Treatment
- What are some related terms associated with atmosphere furnaces? Explore Types for Your Heat Treatment Needs
- Why is it necessary to use an atmosphere furnace with argon gas? Ensure Precise Alloy Austenitization & Protection
- How is a reducing atmosphere utilized in metal processing? Prevent Oxidation and Enhance Metal Quality
- What is the function of an atmosphere tube furnace in activated carbon treatment? Precision Thermal Control



















