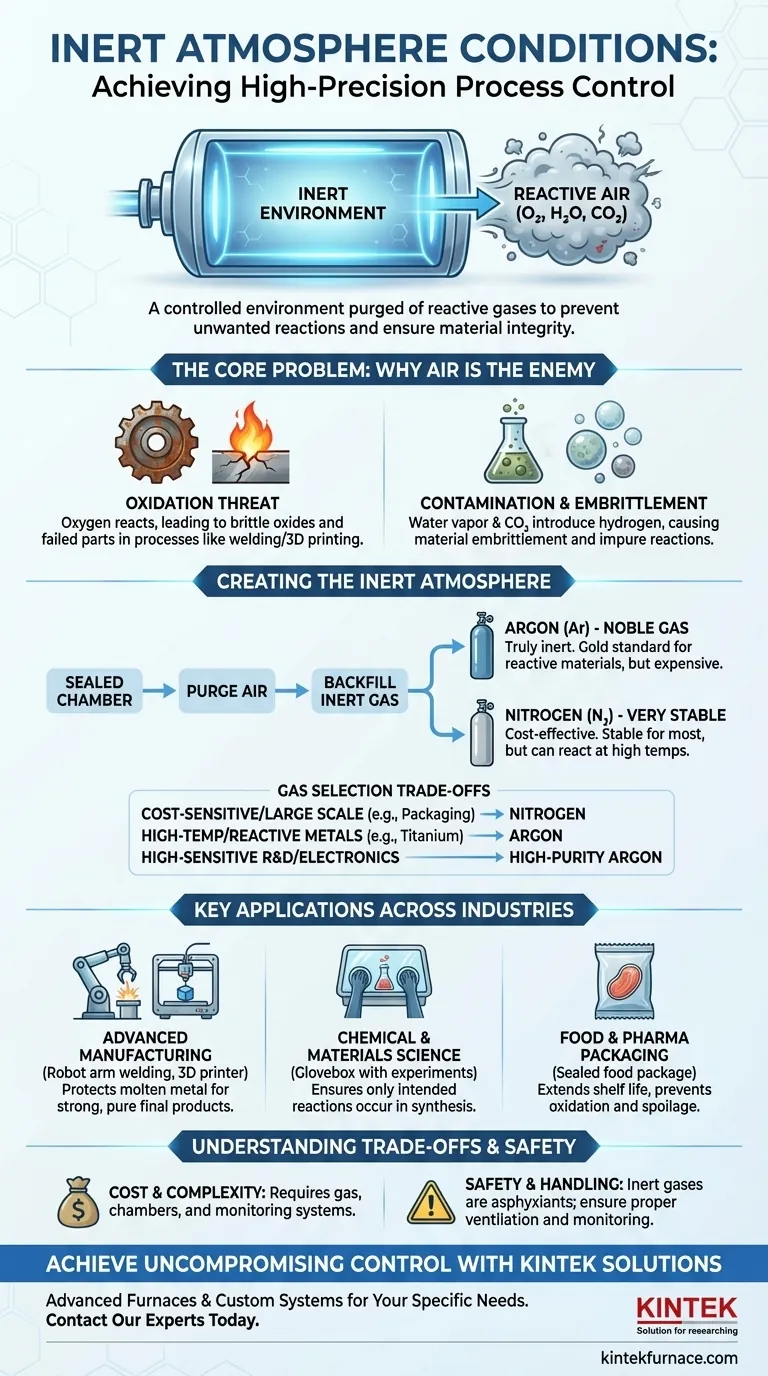
In any high-precision process, an inert atmosphere is a controlled environment that has been purged of reactive gases like oxygen, carbon dioxide, and water vapor. It is then filled with a chemically inactive (inert) gas, typically argon or nitrogen. This is done to prevent unwanted chemical reactions, such as oxidation, which would otherwise contaminate materials and compromise the integrity of the process.
The fundamental purpose of an inert atmosphere is not simply to fill a space, but to actively displace and remove reactive atmospheric gases. This protects sensitive materials and guarantees the outcome of a delicate chemical or physical process.

The Core Problem: Why Air is an Enemy to Precision
Normal air is a mixture of gases that is highly reactive, especially under conditions involving heat or sensitive chemicals. For many technical applications, allowing processes to occur in open air is a non-starter.
The Threat of Oxidation
Oxygen is the most significant challenge. It is highly reactive and seeks to bond with other elements, a process called oxidation.
A familiar example is iron rusting, but this process accelerates dramatically with heat. In processes like welding or metal 3D printing, molten metal exposed to oxygen would instantly form brittle oxides, leading to a weak and failed part.
Contamination from Other Gases
Beyond oxygen, other gases in the air can cause problems. Water vapor can introduce hydrogen that leads to material embrittlement, and carbon dioxide can also interfere with specific chemical reactions, leading to impure results.
Creating and Using an Inert Environment
An inert atmosphere is created by first placing the material or process inside a sealed chamber. This chamber is then purged of air and backfilled with a specific inert gas.
The Common Inert Gases
Argon (Ar) is a noble gas, making it truly chemically inert under almost all conditions. It is the gold standard for protecting highly reactive materials, but it is also more expensive.
Nitrogen (N₂) is very stable and behaves as an inert gas in most applications. Because it is significantly cheaper than argon, it is widely used. However, it is not technically inert and can react with certain metals at very high temperatures.
Key Applications Across Industries
The need to prevent unwanted reactions is critical in numerous fields.
Advanced Manufacturing (Welding & 3D Printing) In processes like TIG welding or powder bed fusion 3D printing, metals are melted at extreme temperatures. An inert gas shield is essential to protect the molten metal pool from oxygen, ensuring a strong, pure, and non-brittle final product.
Chemical and Materials Science When synthesizing chemicals or performing high-temperature heat treatments, the presence of oxygen or water can ruin the experiment. A controlled inert atmosphere in a glovebox or furnace ensures that the only reactions occurring are the ones intended by the scientist.
Food and Pharmaceutical Packaging To extend shelf life and prevent spoilage, food products are often packaged in a "modified atmosphere." By replacing oxygen with nitrogen, producers can slow the growth of microbes and prevent the oxidation that causes food to go stale.
Understanding the Trade-offs
While essential, implementing an inert atmosphere introduces its own set of challenges and costs that must be managed.
Cost and Complexity
Maintaining an inert environment is expensive. The cost includes the ongoing purchase of high-purity gases, as well as the initial investment in sealed chambers (like gloveboxes or process chambers) and the systems required to monitor and control gas purity.
Gas Selection is Critical
The choice between nitrogen and argon is a crucial trade-off. Using nitrogen is more economical, but it can react with reactive metals like titanium at high temperatures to form nitrides, which can alter the material's properties. In such cases, the higher cost of argon is necessary.
Safety and Handling
Inert gases are asphyxiants. While they are not toxic, they displace oxygen. A leak in a poorly ventilated area can create a serious breathing hazard for personnel, requiring strict safety protocols and oxygen monitoring.
Making the Right Choice for Your Goal
Your choice of inert gas and the strictness of your atmospheric control depend entirely on your material, your process, and your budget.
- If your primary focus is cost-sensitive, large-scale applications (like food packaging or steel welding): Nitrogen is almost always the most practical and economical choice.
- If your primary focus is high-temperature processing of reactive metals (like titanium or aluminum): Argon is the superior, non-reactive choice required to avoid material contamination.
- If your primary focus is highly sensitive lab research or electronics manufacturing: High-purity argon is the standard for guaranteeing a truly inactive environment with no unwanted side reactions.
Ultimately, mastering inert atmosphere conditions is fundamental to achieving control, quality, and repeatability in any advanced technical process.
Summary Table:
| Aspect | Description |
|---|---|
| Definition | A controlled environment purged of reactive gases (O₂, CO₂, H₂O) and filled with inert gas (e.g., Ar, N₂). |
| Primary Goal | Prevent unwanted chemical reactions like oxidation to ensure process integrity and material purity. |
| Common Gases | Argon (Ar): Truly inert, ideal for reactive metals. Nitrogen (N₂): Cost-effective for many applications. |
| Key Applications | Advanced manufacturing (welding, 3D printing), chemical synthesis, heat treatment, food/pharma packaging. |
Achieve Uncompromising Process Control with KINTEK Solutions
Mastering inert atmosphere conditions is critical for the success of high-precision processes in manufacturing and R&D. The right equipment is the foundation of this control.
Leveraging exceptional R&D and in-house manufacturing, KINTEK provides diverse laboratories and production facilities with advanced high-temperature furnace and reactor solutions. Our product line, including Tube Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is complemented by our strong deep customization capability to precisely meet your unique atmospheric control requirements.
Whether you are working with reactive metals, sensitive chemicals, or advanced materials, we can help you design a system that delivers the purity and repeatability your work demands.
Ready to eliminate contamination and guarantee your results? Contact our experts today to discuss your specific needs and how our tailored solutions can benefit your process.
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What are the primary inert gases used in vacuum furnaces? Optimize Your Heat Treatment Process
- How do argon and nitrogen protect samples in vacuum furnaces? Optimize Your Thermal Process with the Right Gas
- What are the key features of an atmosphere box furnace? Unlock Precise Heat Processing in Controlled Environments
- How does the pressure range change under vacuum conditions in an atmosphere box furnace? Explore Key Shifts for Material Processing
- How does a mixed gas flow control system maintain stability during high-temperature nitriding? Precision Gas Ratios



















