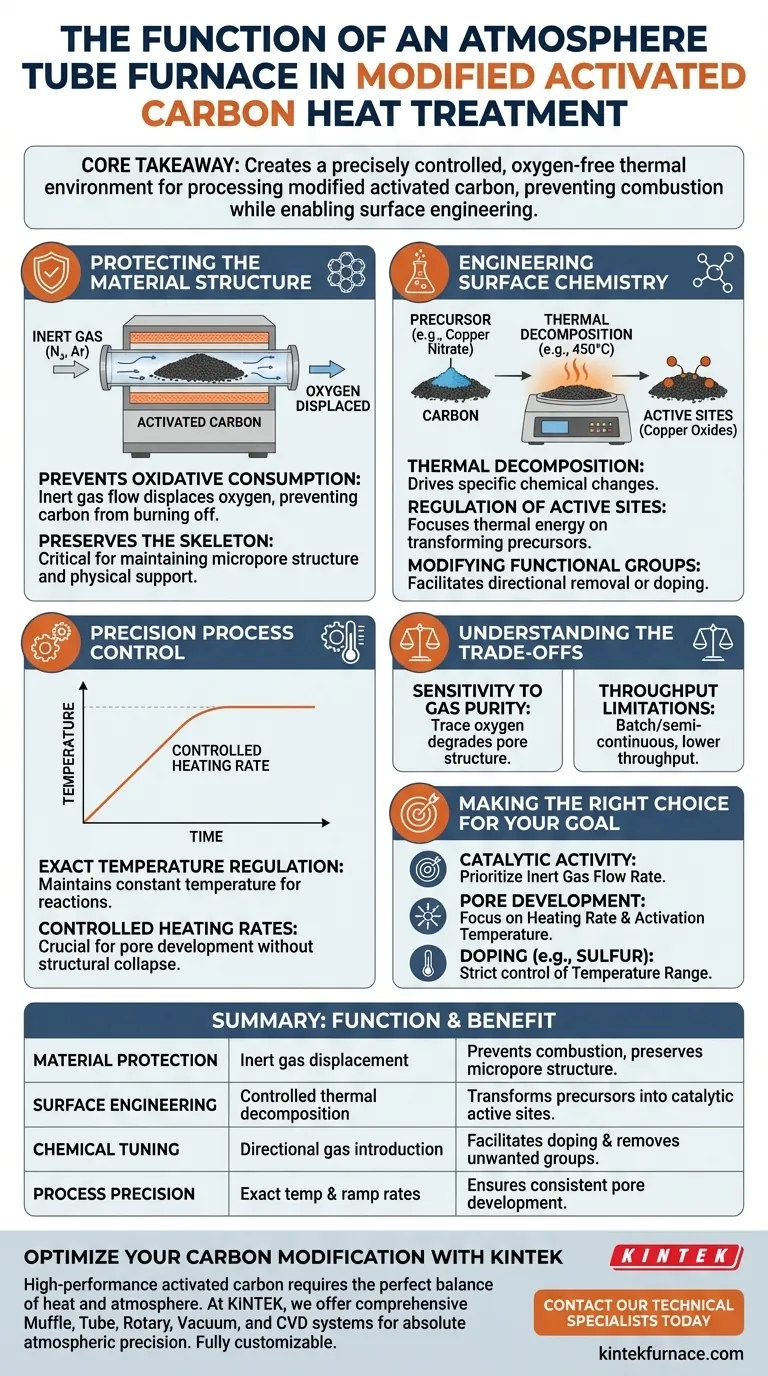
The primary function of an atmosphere tube furnace is to create a precisely controlled, oxygen-free thermal environment for processing modified activated carbon. By maintaining a continuous flow of inert gas (typically nitrogen) during high-temperature treatment, it facilitates the thermal decomposition of chemical precursors into active sites while strictly preventing the activated carbon substrate from burning away.
Core Takeaway Heat treating activated carbon is a delicate balancing act: you must heat the material enough to alter its chemistry without destroying its structure. The atmosphere tube furnace solves this by replacing reactive air with inert gas, ensuring that surface precursors (like copper nitrate) decompose into active oxides, while the carbon skeleton remains intact and protected from oxidative consumption.

Protecting the Material Structure
The most immediate risk when heating activated carbon is combustion. The tube furnace mitigates this through rigorous atmospheric control.
Preventing Oxidative Consumption
Activated carbon is highly susceptible to oxidation at high temperatures. Without a protective atmosphere, the carbon substrate would simply burn off, destroying the material. The tube furnace utilizes an inert gas flow, such as nitrogen or argon, to displace oxygen.
Preserving the Skeleton
This oxygen-free environment is critical for maintaining the material's structural integrity. It preserves the carbon's micropore structure and skeleton, which serves as the physical support for any loaded chemical agents.
Engineering Surface Chemistry
Beyond simple protection, the furnace acts as a chemical reactor that modifies the surface properties of the carbon.
Thermal Decomposition of Precursors
For modified activated carbon, the furnace drives specific chemical changes. A primary example is copper nitrate-loaded carbon. At temperatures around 450°C, the furnace ensures the nitrate decomposes into highly active copper oxides (CuO or Cu2O).
Regulation of Active Sites
The inert atmosphere allows for the precise regulation of these surface reactions. Because the carbon substrate is not being consumed by oxygen, the thermal energy focuses solely on transforming the precursor materials into catalytic active sites.
Modifying Functional Groups
The furnace can also be used to fine-tune the carbon's intrinsic chemistry. By introducing specific gases or controlling the temperature profile, it facilitates the directional removal of oxygen-containing functional groups or the doping of elements like sulfur into the carbon framework.
Precision Process Control
The "tube" design of the furnace allows for exact control over the thermal profile, which is essential for consistent results.
Exact Temperature Regulation
Different modifications require specific thermal activation points. Whether it is decomposing nitrates at 450°C or carbonizing precursors at 850°C, the furnace maintains the constant temperature duration necessary for these reactions to complete.
Controlled Heating Rates
The rate at which temperature increases is as important as the final temperature. The furnace allows for specific heating rates (e.g., 80 °C/min), which helps control the development of pores and the reorganization of the carbon skeleton without causing structural collapse.
Understanding the Trade-offs
While essential for high-performance materials, using an atmosphere tube furnace introduces specific complexities.
Sensitivity to Gas Purity
The process is entirely dependent on the quality of the atmosphere. Even trace amounts of oxygen in the nitrogen or argon flow can lead to partial oxidation of the carbon, degrading its pore structure and reducing its final surface area.
Throughput Limitations
Tube furnaces are typically batch or semi-continuous processing units. While they offer superior control for high-value modified carbons, they generally have lower throughput compared to continuous industrial rotary kilns used for lower-grade materials.
Making the Right Choice for Your Goal
To maximize the effectiveness of an atmosphere tube furnace, tailor your process parameters to your specific material objectives.
- If your primary focus is Catalytic Activity: Prioritize the inert gas flow rate to ensure the complete decomposition of precursors (like copper nitrate) into oxides without damaging the support structure.
- If your primary focus is Pore Development: Focus on the heating rate and activation temperature (e.g., with KOH at 800°C) to etch the carbon skeleton and expand surface area.
- If your primary focus is Doping (e.g., Sulfur): strict control of the temperature range (500-900°C) is required to form stable chemical bonds within the framework.
Success depends not just on heating the material, but on strictly controlling the atmosphere to define exactly what reacts and what remains.
Summary Table:
| Function Category | Key Mechanism | Benefit for Activated Carbon |
|---|---|---|
| Material Protection | Inert gas displacement (Nitrogen/Argon) | Prevents carbon combustion and preserves micropore structure. |
| Surface Engineering | Controlled thermal decomposition | Transforms precursors (e.g., copper nitrate) into catalytic active sites. |
| Chemical Tuning | Directional gas introduction | Facilitates doping (Sulfur/Nitrogen) and removes unwanted functional groups. |
| Process Precision | Exact temperature & ramp rates | Ensures consistent pore development and structural integrity. |
Optimize Your Carbon Modification with KINTEK
High-performance activated carbon requires the perfect balance of heat and atmosphere. At KINTEK, we understand that even trace oxygen can ruin your material's surface area. Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems designed for absolute atmospheric precision.
Whether you are scaling up catalytic production or engineering next-generation doping processes, our laboratory high-temp furnaces are fully customizable to meet your unique research and production needs.
Ready to achieve superior thermal control?
Contact our technical specialists today to find the ideal furnace solution for your laboratory.
Visual Guide
References
- Bin Liu, Songlin Zuo. Significance of micropores for the removal of hydrogen sulfide from oxygen-free gas streams by activated carbon. DOI: 10.1515/htmp-2025-0085
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- What are the operational advantages of using a controlled atmosphere furnace? Boost Quality and Efficiency in Heat Treatment
- How does a controlled atmosphere furnace improve product quality and consistency? Master Precise Heat Treatment for Superior Results
- What role does a vacuum or atmosphere tube furnace play in the sintering process of Al6061/B4C composites?
- What is the mechanism by which a reducing atmosphere improves Mn-Zn ferrite performance? Unlocking Magnetic Excellence
- What provides inert atmosphere for high-temperature metallurgical process? Prevent Oxidation with Argon & More
- What safety features are typically included in controlled atmosphere furnaces? Ensure Safe Operation with Advanced Protection
- How does the temperature control system work in the box type annealing atmosphere furnace? Achieve Precise Thermal Management
- How does the box type annealing atmosphere furnace generate heat? Master Precise Temperature Control for Your Lab



















