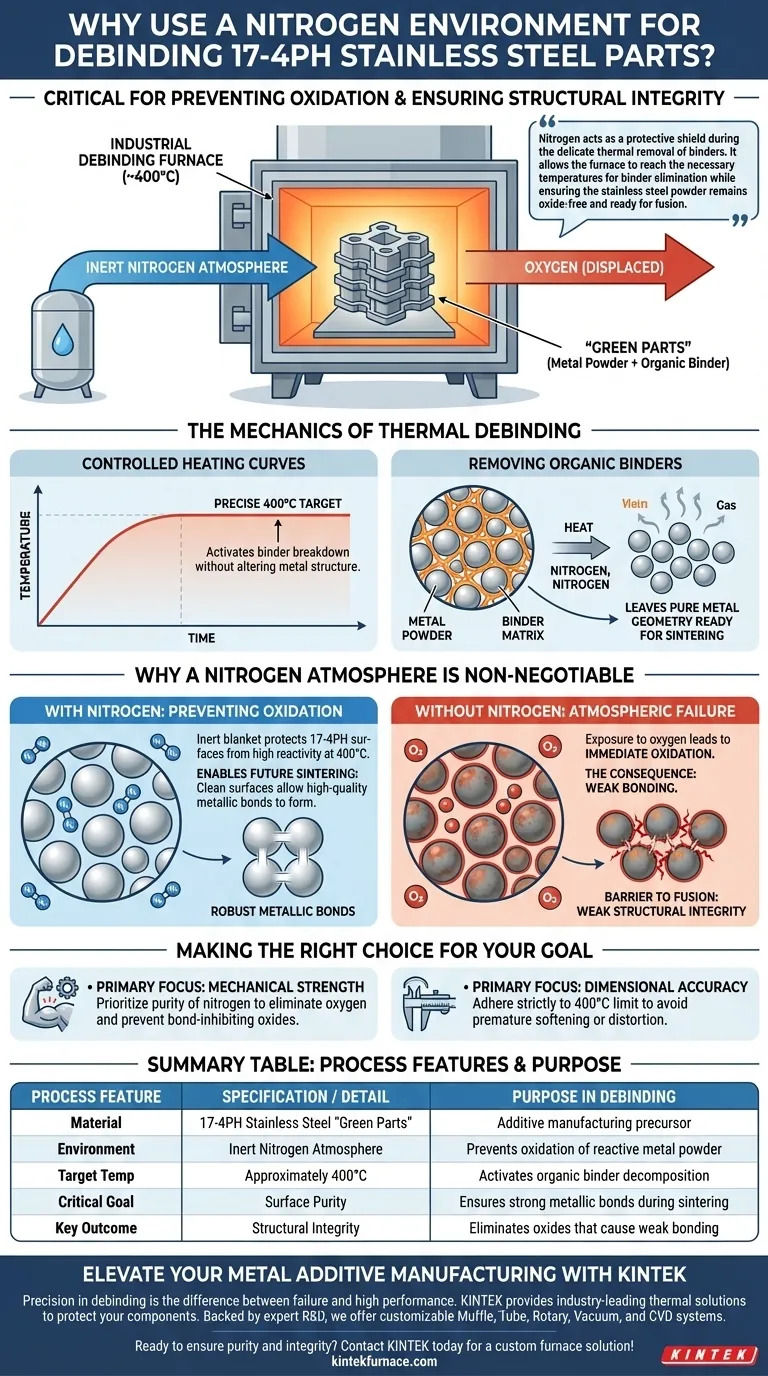
The use of a nitrogen environment in an industrial debinding furnace is the critical factor in preventing oxidation while preparing 17-4PH stainless steel parts for final strengthening. Operating at approximately 400°C, the furnace strictly controls the thermal removal of organic binders from "green parts." This inert atmosphere ensures that the metal powder remains chemically pure, allowing for the formation of robust metallic bonds during the subsequent sintering phase.
Nitrogen acts as a protective shield during the delicate thermal removal of binders. It allows the furnace to reach the necessary temperatures for binder elimination while ensuring the stainless steel powder remains oxide-free and ready for fusion.
The Mechanics of Thermal Debinding
Controlled Heating Curves
The primary function of the industrial furnace in this context is to execute a precise heating curve.
Rather than rapid heating, the temperature is carefully raised to 400°C. This specific temperature is targeted to activate the breakdown of binders without altering the metal's structure.
Removing Organic Binders
Additive manufacturing often uses organic binders to hold metal powder together in a shape known as a "green part."
The furnace heat causes these binders to decompose or evaporate. This leaves behind the pure metal geometry, ready for the final fusing process.
Why a Nitrogen Atmosphere is Non-Negotiable
Preventing Oxidation
At 400°C, 17-4PH stainless steel powder is highly reactive to oxygen.
If exposed to standard air at this temperature, the metal particles would immediately oxidize. The nitrogen environment displaces oxygen, creating an inert blanket that protects the metal surfaces from chemical degradation.
Enabling Future Sintering
The ultimate goal of 3D printing metal is to sinter—or fuse—particles into a solid mass.
High-quality metallic bonds can only form between clean metal surfaces. If the particles oxidize during debinding, those oxides act as a barrier, preventing the particles from fusing correctly in the next stage.
The Consequence of Atmospheric Failure
The Risk of Weak Bonding
It is critical to understand that debinding is not just about cleaning the part; it is about preserving surface chemistry.
Failure to maintain a nitrogen environment leads to surface contamination. This results in weak structural integrity because the metal particles will physically touch but fail to chemically bond during sintering.
Making the Right Choice for Your Goal
To ensure the success of your 17-4PH stainless steel manufacturing process, consider these priorities:
- If your primary focus is mechanical strength: Prioritize the purity of the nitrogen atmosphere to completely eliminate oxygen and prevent bond-inhibiting oxides.
- If your primary focus is dimensional accuracy: Adhere strictly to the 400°C temperature limit to ensure binder removal occurs without prematurely softening or distorting the metal powder.
Control the atmosphere today to ensure the strength of the part tomorrow.
Summary Table:
| Process Feature | Specification/Detail | Purpose in Debinding |
|---|---|---|
| Material | 17-4PH Stainless Steel | Additive manufacturing "green parts" |
| Environment | Inert Nitrogen Atmosphere | Prevents oxidation of reactive metal powder |
| Target Temp | Approximately 400°C | Activates organic binder decomposition |
| Critical Goal | Surface Purity | Ensures strong metallic bonds during sintering |
| Key Outcome | Structural Integrity | Eliminates oxides that cause weak bonding |
Elevate Your Metal Additive Manufacturing with KINTEK
Precision in the debinding phase is the difference between a failed part and high-performance engineering. KINTEK provides industry-leading thermal solutions designed to protect your 17-4PH stainless steel components from oxidation.
Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable for your specific high-temperature lab and industrial needs.
Ready to ensure the chemical purity and structural integrity of your 3D-printed parts? Contact KINTEK today for a custom furnace solution!
Visual Guide
References
- Suhair Ghazi Mahdi. Comparative Study of Additive Manufacturing Techniques and Post-Processing on Microstructure and Properties of 17-4PH Stainless Steel and GRCop-42 Copper Alloy: Sintering Optimization vs Recrystallization Annealing. DOI: 10.22399/ijcesen.2657
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the purpose of sealing mechanisms in atmosphere furnaces? Ensure Process Purity and Safety
- What temperature control capabilities does a box type atmosphere furnace have? Achieve Precise Thermal Management for Your Lab
- What is a program-controlled atmosphere furnace? Master Precise Heat Treatment for Advanced Materials
- How does an oxygen atmosphere furnace help optimize the optical performance of SiO2 microarchitectures? Enhancing Clarity
- Why is a water-cooling spray system implemented in annealing? Maximize Production Throughput & Material Quality
- Why is a nitrogen environment necessary for Cu13Se52Bi35 thin film annealing? Protect Your Material Purity
- What is the role of argon in atmosphere furnaces? Ensure Purity and Prevent Oxidation in Heat Treatment
- What are the common gases and vapors used in furnace atmospheres and their roles? Optimize Your Heat Treatment Process



















