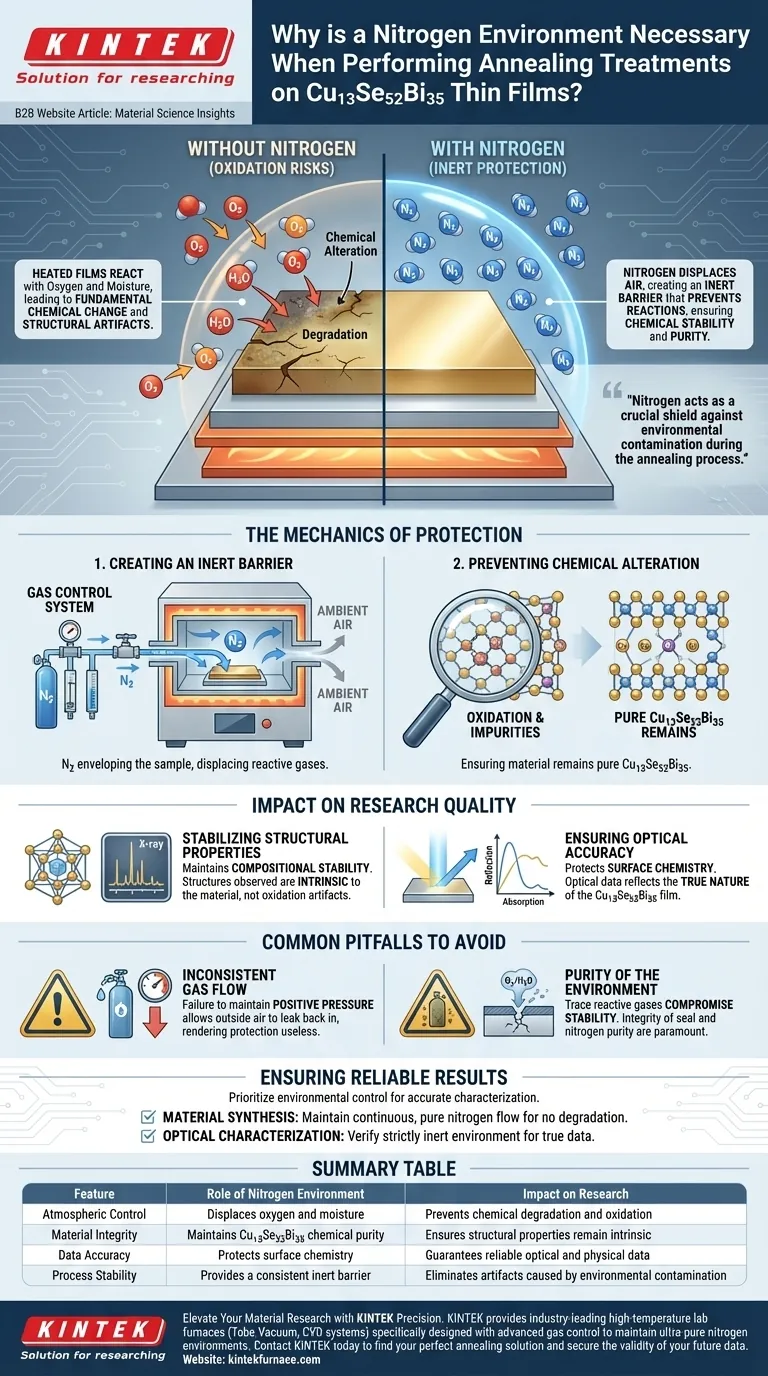
A nitrogen environment is strictly necessary when annealing Cu13Se52Bi35 thin films to establish an inert protective barrier. By displacing standard air, this environment prevents the heated films from reacting with oxygen or atmospheric moisture. This isolation preserves the film's chemical stability during the high-temperature treatment.
Nitrogen acts as a crucial shield against environmental contamination during the annealing process. Without this inert atmosphere, the material would degrade through oxidation, compromising the accuracy of subsequent structural and optical research.

The Mechanics of Protection
Creating an Inert Barrier
When Cu13Se52Bi35 thin films are subjected to heat, they become highly reactive to their surroundings. An annealing furnace equipped with a gas control system is used to introduce nitrogen into the chamber. This effectively displaces the ambient air, enveloping the sample in a non-reactive gas.
Preventing Chemical Alteration
The primary danger during annealing is exposure to oxygen and moisture naturally present in the air. If the films interact with these elements while heated, their chemical composition will fundamentally change. Nitrogen prevents these unwanted reactions, ensuring the material remains pure Cu13Se52Bi35.
Impact on Research Quality
Stabilizing Structural Properties
Research into the physical structure of thin films relies on the material remaining consistent throughout the experiment. By using nitrogen to maintain compositional stability, researchers can be confident that the structures they observe are intrinsic to the material, not artifacts of oxidation.
Ensuring Optical Accuracy
The optical properties of a thin film are heavily dependent on its surface chemistry and purity. Any reaction with the atmosphere could alter how the film absorbs or reflects light. The nitrogen environment guarantees that the optical data collected reflects the true nature of the Cu13Se52Bi35 film.
Common Pitfalls to Avoid
Inconsistent Gas Flow
Simply introducing nitrogen is not enough; the environment must remain stable. If the gas control system fails to maintain positive pressure or adequate flow, outside air may leak back into the chamber. This breach renders the protective atmosphere useless.
Purity of the Environment
While nitrogen is inert, the effectiveness of the process depends on the total exclusion of reactive gases. Even trace amounts of oxygen or moisture leaking into the furnace can compromise the stability of the chemical composition. The integrity of the seal and the purity of the nitrogen source are paramount.
Ensuring Reliable Results
To achieve accurate characterization of Cu13Se52Bi35 thin films, you must prioritize environmental control during thermal treatment.
- If your primary focus is Material Synthesis: Ensure your gas control system maintains a continuous, pure nitrogen flow to prevent irreversible chemical degradation.
- If your primary focus is Optical Characterization: Verify that the annealing environment was strictly inert to ensure your data reflects the true properties of the film, not surface oxides.
Control the atmosphere today to ensure the validity of your data tomorrow.
Summary Table:
| Feature | Role of Nitrogen Environment | Impact on Research |
|---|---|---|
| Atmospheric Control | Displaces oxygen and moisture | Prevents chemical degradation and oxidation |
| Material Integrity | Maintains Cu13Se52Bi35 chemical purity | Ensures structural properties remain intrinsic |
| Data Accuracy | Protects surface chemistry | Guarantees reliable optical and physical data |
| Process Stability | Provides a consistent inert barrier | Eliminates artifacts caused by environmental contamination |
Elevate Your Material Research with KINTEK Precision
Don't let oxidation compromise your thin film characterization. KINTEK provides industry-leading high-temperature lab furnaces—including Tube, Vacuum, and CVD systems—specifically designed with advanced gas control to maintain the ultra-pure nitrogen environments your research demands.
Backed by expert R&D and precision manufacturing, our customizable solutions ensure your materials maintain their chemical stability every time. Contact KINTEK today to find your perfect annealing solution and secure the validity of your future data.
Visual Guide
References
- Abduelwhab B. Alwany, Abdulnasser Abdulrahman Alfaqeer. Effect of annealing temperature on the structural and optical properties of vacuum evaporated Cu13Se52Bi35 thin films. DOI: 10.15251/cl.2024.211.99
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the application of box type annealing atmosphere furnace in automotive parts manufacturing? Boost Performance and Efficiency
- How does a retort furnace control the atmosphere? Master Precise Heat Treatment for Superior Results
- What types of chemical processes are facilitated by retort furnaces? Unlock Precise High-Temperature Control
- What role do cooling systems play in retort furnaces? Master Material Properties with Precision Cooling
- Why is a rotameter essential for controlling the atmosphere within an oily sludge pyrolysis reactor? Master Gas Flow Control
- How does an atmosphere protection furnace ensure the quality of CoCrFeNiMn coatings? Optimized Heat Treatment Solutions
- What are the key components of an inert atmosphere furnace? Essential Parts for Contamination-Free Heating
- What function does a flow-gas furnace serve in iron ore reduction? Mastering Lab Gas Delivery and Thermal Sync



















