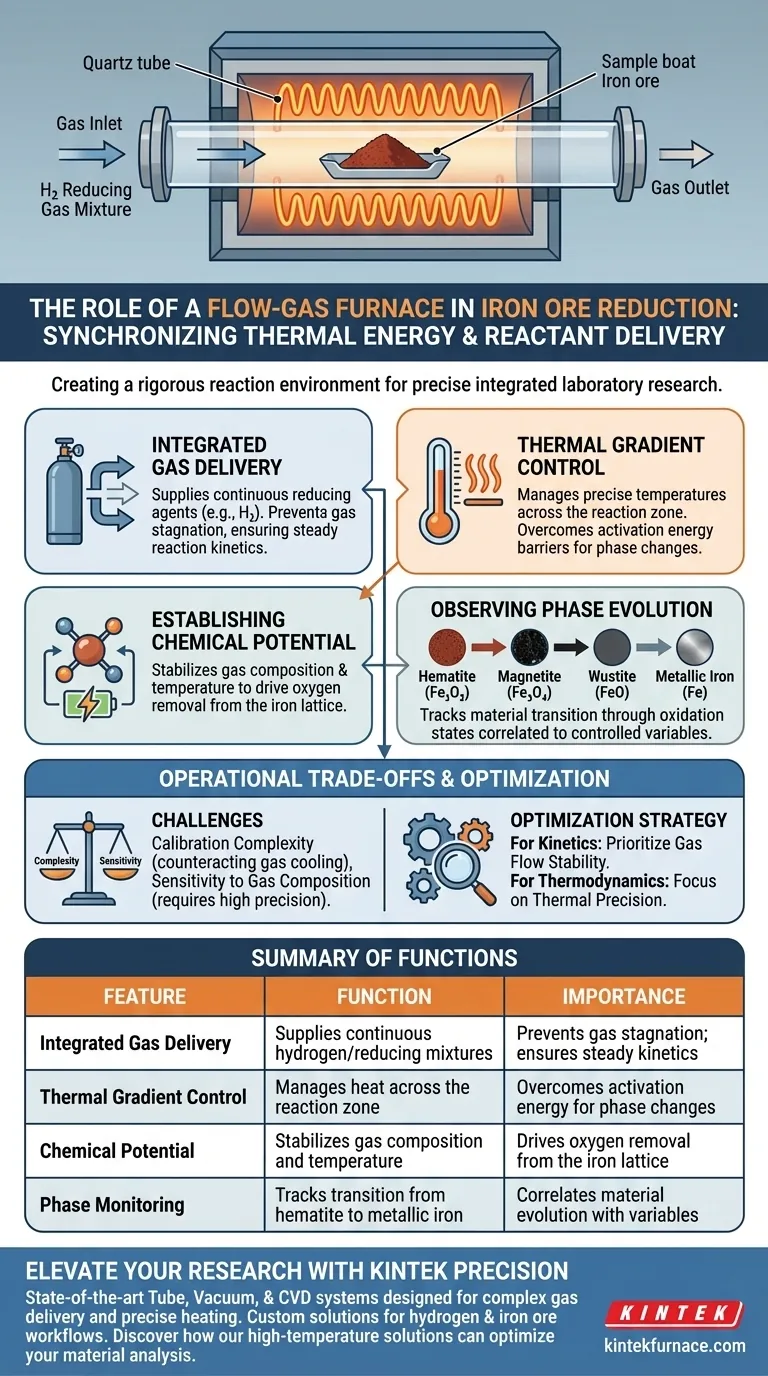
The primary function of a flow-gas furnace in this context is to create a rigorous reaction environment by synchronizing thermal energy with reactant delivery. In integrated laboratory systems, the furnace ensures that reducing gases, such as hydrogen mixtures, flow through the reaction chamber at a constant rate while simultaneously maintaining precise temperature gradients. This integration is essential for simulating the exact conditions required to drive the chemical reduction of iron ore.
The core value of this system lies in its ability to establish a controlled chemical potential. By locking in gas flow and temperature variables, it allows researchers to isolate and observe phase evolution driven specifically by the interplay of thermal changes and gas composition.

Establishing the Reaction Environment
Integrated Gas Delivery
The furnace operates as more than a simple heating element; it acts as an active flow reactor.
It ensures that the reducing agent—typically a specific mixture of hydrogen—is delivered continuously to the sample site.
maintaining a constant flow rate is critical to prevent gas stagnation, which would alter reaction kinetics.
Thermal Gradient Control
Beyond simple heating, the system manages temperature gradients across the reaction zone.
This allows for precise control over the thermal energy supplied to the iron ore.
Specific temperatures are required to overcome activation energy barriers for different reduction stages.
Observing Phase Evolution
Defining Chemical Potential
The simultaneous control of gas composition and temperature creates a specific chemical potential.
This thermodynamic state dictates the driving force for removing oxygen from the iron lattice.
By stabilizing this environment, the system ensures that the reduction process proceeds predictably.
Monitoring Phase Changes
The furnace enables the observation of the material's transition through various oxidation states.
Researchers can track the evolution from hematite to magnetite, wustite, and metallic iron.
These observations can be directly correlated to the controlled variables of temperature and gas mixture.
Understanding Operational Trade-offs
Calibration Complexity
Integrating gas flow with heating introduces significant complexity to the system setup.
The cooling effect of a flowing gas must be counteracted by the heating elements to maintain temperature accuracy.
Poor calibration can lead to thermal gradients that differ from the target setpoints.
Sensitivity to Gas Composition
The system creates an environment highly sensitive to the exact mixture of the reducing gas.
Slight deviations in gas composition can drastically alter the chemical potential.
This requires the gas delivery components to be as precise as the thermal controllers to avoid experimental error.
Optimizing Your Experimental Setup
To maximize the utility of a flow-gas furnace, you must tailor your control strategy to the specific aspect of reduction you are investigating.
- If your primary focus is reaction kinetics: Prioritize the stability of the gas flow rate to ensure that mass transport of the reducing agent is consistent and measurable.
- If your primary focus is thermodynamic stability: Focus on the precision of the thermal gradients to accurately map the boundaries where specific iron phases evolve.
Ultimately, the flow-gas furnace serves as the critical control point where thermodynamics and kinetics intersect, enabling the systematic analysis of iron ore reduction.
Summary Table:
| Feature | Function in Iron Ore Reduction | Importance |
|---|---|---|
| Integrated Gas Delivery | Supplies continuous hydrogen/reducing mixtures | Prevents gas stagnation; ensures steady kinetics |
| Thermal Gradient Control | Manages heat across the reaction zone | Overcomes activation energy for phase changes |
| Chemical Potential | Stabilizes gas composition and temperature | Drives oxygen removal from the iron lattice |
| Phase Monitoring | Tracks transition from hematite to metallic iron | Correlates material evolution with variables |
Elevate Your Iron Ore Research with KINTEK Precision
To achieve accurate phase evolution and thermodynamic stability, your laboratory requires hardware that masters the intersection of kinetics and thermal control. KINTEK provides state-of-the-art Tube, Vacuum, and CVD systems designed to synchronize complex gas delivery with precise heating gradients.
Backed by expert R&D and manufacturing, our systems are fully customizable to handle hydrogen mixtures and specialized iron ore reduction workflows. Contact KINTEK today to discuss your unique experimental needs and discover how our high-temperature solutions can optimize your material analysis.
Visual Guide
References
- Yuzhao Wang, Samuli Urpelainen. In Situ SXRD Study of Phase Transformations and Reduction Kinetics in Iron Ore During Hydrogen-Based High-Temperature Reduction. DOI: 10.1007/s11663-025-03725-2
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the role of calcining beta-zeolite at 750°C? Mastering Phase Transformation for High-Performance Catalysts
- What are the advantages of low vacuum atmosphere furnaces? Boost Efficiency and Cut Costs
- Why is it necessary to perform air atmosphere annealing after sintering Y2O3-YAM composite ceramics?
- What high-temperature processes commonly use argon in furnaces? Essential Guide for Reactive Metals
- How is a protective atmosphere contained in a furnace? Engineered Seals and Positive Pressure Explained
- How does an inert atmosphere furnace work? Master Controlled Heating for Oxidation-Free Results
- How does a reactive furnace atmosphere benefit heat treatment? Enhance Surface Hardness and Wear Resistance
- Why is an inert atmosphere important in heat treatment processes? Prevent Oxidation and Ensure Material Integrity



















