At its core, a reactive furnace atmosphere is a precisely controlled gas mixture that intentionally triggers chemical reactions on a part's surface during heat treatment. Unlike a simple protective atmosphere, its primary purpose is not just to prevent damage but to actively change the material's surface chemistry, thereby enhancing specific properties like hardness and wear resistance.
The crucial distinction to understand is that a reactive atmosphere is not a passive shield but an active engineering tool. It transforms the surface of a component by delivering key chemical elements, turning a standard heat treatment process into a sophisticated surface modification technique.

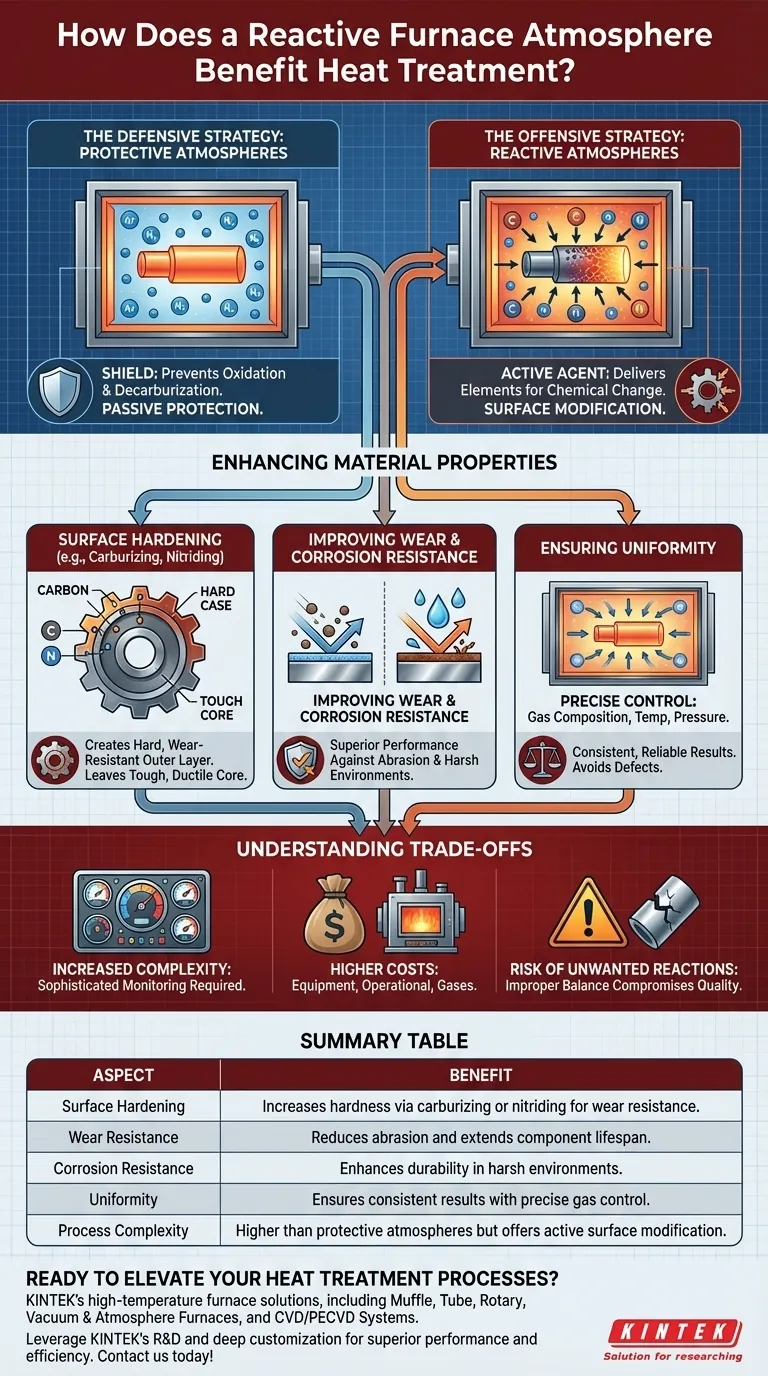
The Dual Role of Atmosphere in Heat Treatment
To grasp the benefit of a reactive atmosphere, one must first understand the two fundamental atmospheric strategies in heat treatment: protection and reaction. High temperatures make metals highly susceptible to chemical changes, and controlling the atmosphere is how we dictate what those changes will be.
The Defensive Strategy: Protective Atmospheres
A protective atmosphere serves as a shield. Its goal is to prevent the hot metal part from reacting with damaging elements in the air, primarily oxygen and water vapor.
This approach prevents common defects like oxidation (scaling) and decarburization (the loss of carbon from the surface of steel), which can compromise a part's integrity and performance. Inert gases like argon or nitrogen are often used for this purpose.
The Offensive Strategy: Reactive Atmospheres
A reactive atmosphere takes an active, or "offensive," approach. It is designed to be the primary agent of change.
This atmosphere acts as a carrier, transporting specific, desirable elements to the part's surface. This induces a controlled chemical reaction that fundamentally alters the surface's composition and microstructure.
How Reactive Atmospheres Enhance Material Properties
By using the atmosphere as a delivery mechanism for chemical change, engineers can achieve significant improvements in a material's final characteristics.
Delivering Elements for Surface Hardening
The most common application is surface hardening, also known as case hardening. By introducing carbon (carburizing) or nitrogen (nitriding) into the atmosphere, these elements diffuse into the surface of a steel part.
This creates an extremely hard, wear-resistant outer "case" while leaving the inner "core" of the material tougher and more ductile. The result is a component that can withstand surface abrasion while resisting fracture from impact.
Improving Wear and Corrosion Resistance
The chemical changes imparted by a reactive atmosphere directly lead to superior performance. A harder surface naturally has better wear resistance.
Furthermore, introducing elements like nitrogen can also significantly improve a material's corrosion resistance, extending the component's service life in harsh environments.
Ensuring Uniformity Through Precise Control
Modern atmosphere furnaces provide the precise control necessary for these reactions to succeed. Regulating gas composition, temperature, and pressure ensures the chemical reaction is uniform across the entire part surface.
This precision is critical for producing consistent, reliable results and avoiding defects that could arise from an uncontrolled or imbalanced reaction.
Understanding the Trade-offs
While powerful, employing a reactive atmosphere introduces complexities that are important to acknowledge.
Increased Process Complexity
Managing a reactive gas mixture is inherently more complex than using an inert gas or air. It requires sophisticated monitoring and control systems to maintain the precise chemical balance needed for the desired reaction.
Higher Equipment and Operational Costs
Furnaces capable of handling reactive atmospheres, along with the cost of the gases themselves, represent a higher investment. The technical oversight required to run these processes safely and effectively also adds to the operational cost.
The Risk of Unwanted Reactions
If not controlled perfectly, a reactive atmosphere can cause problems. An improper gas balance can lead to the formation of unwanted microstructures, embrittlement, or other surface defects that compromise the part's quality.
Making the Right Choice for Your Goal
Selecting the correct furnace atmosphere is a critical decision that depends entirely on the desired outcome for the material.
- If your primary focus is preventing surface damage like oxidation during annealing: A protective (inert) atmosphere is the most direct and cost-effective solution.
- If your primary focus is significantly increasing surface hardness and wear resistance: A reactive atmosphere for processes like carburizing or nitriding is essential.
- If your primary focus is simple stress-relief on a non-critical part: A less complex atmosphere, or even air for certain alloys, may be sufficient and more economical.
Ultimately, choosing a reactive atmosphere means you are deliberately using chemistry as a tool to engineer a superior material surface.
Summary Table:
| Aspect | Benefit |
|---|---|
| Surface Hardening | Increases hardness via carburizing or nitriding for wear resistance. |
| Wear Resistance | Reduces abrasion and extends component lifespan. |
| Corrosion Resistance | Enhances durability in harsh environments. |
| Uniformity | Ensures consistent results with precise gas control. |
| Process Complexity | Higher than protective atmospheres but offers active surface modification. |
Ready to elevate your heat treatment processes with advanced furnace solutions? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide diverse laboratories with high-temperature furnace solutions tailored to your needs. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all supported by strong deep customization capabilities to precisely meet unique experimental requirements. Whether you're aiming for enhanced surface hardening or improved material properties, our expertise ensures optimal results. Contact us today to discuss how we can help you achieve superior performance and efficiency in your lab!
Visual Guide
Related Products
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What are the benefits of inert atmosphere heat treating? Prevent Oxidation and Preserve Material Integrity
- Why is moisture control critical in inert atmosphere heat treating? Prevent Oxidation and Ensure Material Integrity
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance
- What industries commonly use inert atmosphere heat treating? Key Applications in Military, Automotive, and More



















