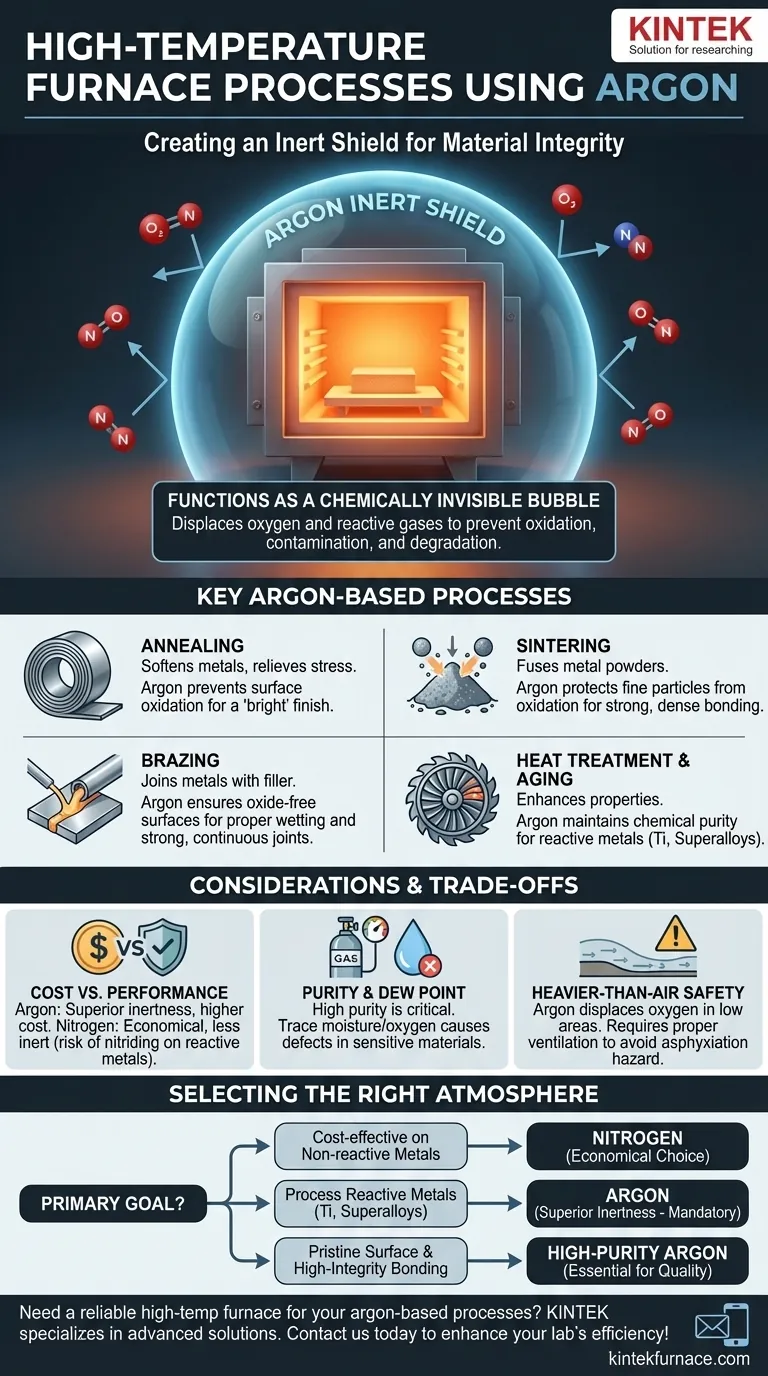
In high-temperature furnaces, argon is most commonly used for processes like annealing, sintering, brazing, and the heat treatment of reactive or high-value alloys. It functions as an inert shield, displacing oxygen and other reactive gases to prevent oxidation, contamination, and degradation of the material being processed. This ensures the final product maintains its intended chemical purity, structural integrity, and surface finish.
The core purpose of using argon in a furnace is not to participate in the process, but to prevent unwanted reactions. Think of it as creating a chemically invisible bubble around the material, protecting it from the damaging effects of air at extreme temperatures.

The Fundamental Role of Argon: Creating an Inert Shield
High-temperature processing is fundamentally about controlling material properties through heat. However, heat also dramatically accelerates chemical reactions, primarily with the components of ambient air—oxygen and nitrogen.
Why an Inert Atmosphere is Critical
As metals heat up, their atoms become more mobile and reactive. When exposed to air, this results in oxidation (forming oxides, like rust or scale) and sometimes nitriding (forming nitrides). These reactions alter the surface, chemistry, and mechanical properties of the material in undesirable ways.
Preventing Oxidation and Discoloration
Argon is a noble gas, meaning it is chemically inert and will not react with other elements, even at high temperatures. By flooding the furnace chamber, argon displaces oxygen, effectively eliminating the risk of oxidation.
This is the principle behind bright annealing, where the goal is to soften a metal without creating the dark, scaly oxide layer that would otherwise form, preserving a clean, "bright" surface.
Protecting Reactive and High-Purity Metals
For highly reactive metals like titanium, zirconium, and certain nickel-based superalloys, protection is even more critical. These materials can be ruined by contact with oxygen or even nitrogen at high temperatures.
Argon provides a truly inert environment, ensuring these expensive and performance-critical materials, often used in aerospace and medical applications, meet their stringent quality standards.
A Breakdown of Key Argon-Based Furnace Processes
Different heat treatment processes leverage argon's protective properties to achieve specific outcomes.
Annealing
This process softens metals and relieves internal stresses by heating and then slowly cooling them. Using an argon atmosphere prevents surface oxidation, which is especially important for parts that require a clean finish or will undergo further processing.
Sintering
Sintering involves heating compacted metal powders to just below their melting point, causing the particles to fuse together. Argon is crucial here to prevent the oxidation of the vast surface area of the fine powders, which would inhibit proper bonding and result in a weak, low-density part.
Brazing
Brazing joins two or more metal items by melting and flowing a filler metal into the joint. An argon atmosphere prevents oxides from forming on both the base materials and the filler metal, ensuring the braze alloy can wet the surfaces properly and create a strong, clean, and continuous bond.
Aging and Heat Treatment
Processes like age hardening are used to increase the strength of alloys. These treatments rely on precise time and temperature controls to develop a specific internal microstructure. Argon ensures that the material's chemistry is not altered by surface reactions during this critical phase.
Understanding the Trade-offs
While highly effective, argon is not the only option for creating a controlled atmosphere, and its use involves specific considerations.
Cost vs. Performance
Argon is generally more expensive than nitrogen, which is often used as a cheaper alternative for an inerting atmosphere. However, nitrogen is not truly inert for all materials. At high temperatures, it can react with metals like titanium and some stainless steels to form brittle nitrides.
The choice between argon and nitrogen is a classic engineering trade-off: argon provides superior protection for sensitive materials, while nitrogen is a cost-effective solution for less reactive metals.
Purity and Dew Point
The effectiveness of the argon shield depends on its purity. Even trace amounts of oxygen or water vapor (measured by dew point) in the argon supply can be enough to cause discoloration or minor oxidation on sensitive materials. High-purity argon is often required for the most demanding applications.
Heavier-Than-Air Properties
Argon is about 38% denser than air. This is an advantage inside a furnace, as it can effectively settle and displace the lighter ambient air. However, it also presents a safety consideration, as it can displace oxygen in enclosed or low-lying areas, creating an asphyxiation hazard that requires proper ventilation and monitoring.
Making the Right Choice for Your Goal
Selecting the correct furnace atmosphere is critical to achieving the desired material properties and finish quality.
- If your primary focus is cost-effectiveness on non-reactive metals: Nitrogen is often a sufficient and more economical choice for materials like plain carbon steels.
- If your primary focus is processing highly reactive metals (e.g., titanium, superalloys): Argon's superior inertness is non-negotiable to prevent embrittlement and guarantee material integrity.
- If your primary focus is achieving a pristine, oxide-free surface finish: High-purity argon is the best choice to ensure a clean, bright result without discoloration.
- If your primary focus is high-integrity brazing or sintering: An argon atmosphere is essential to prevent oxides that would interfere with bonding and compromise the strength of the final part.
Ultimately, the decision to use argon is driven by the material's sensitivity to atmospheric reaction and the final quality requirements of the component.
Summary Table:
| Process | Key Use of Argon | Common Materials |
|---|---|---|
| Annealing | Prevents oxidation for clean surface finish | Reactive and high-value alloys |
| Sintering | Protects metal powders from oxidation | Fine metal powders |
| Brazing | Ensures oxide-free bonding for strong joints | Various metals with filler alloys |
| Heat Treatment | Maintains chemical purity and microstructure | Titanium, zirconium, superalloys |
Need a reliable high-temperature furnace for your argon-based processes? KINTEK specializes in advanced solutions like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. With our strong R&D and in-house manufacturing, we offer deep customization to meet your unique experimental needs for reactive metals and high-purity applications. Contact us today to enhance your lab's efficiency and material quality!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What are the development prospects of atmosphere box furnaces in the aerospace industry? Unlock Advanced Material Processing for Aerospace Innovation
- What are the key features of an atmosphere box furnace? Unlock Precise Heat Processing in Controlled Environments
- How does a mixed gas flow control system maintain stability during high-temperature nitriding? Precision Gas Ratios
- What is an atmosphere protection muffle furnace? Unlock Precise Heat Treatment in Controlled Environments
- How does the pressure range change under vacuum conditions in an atmosphere box furnace? Explore Key Shifts for Material Processing



















