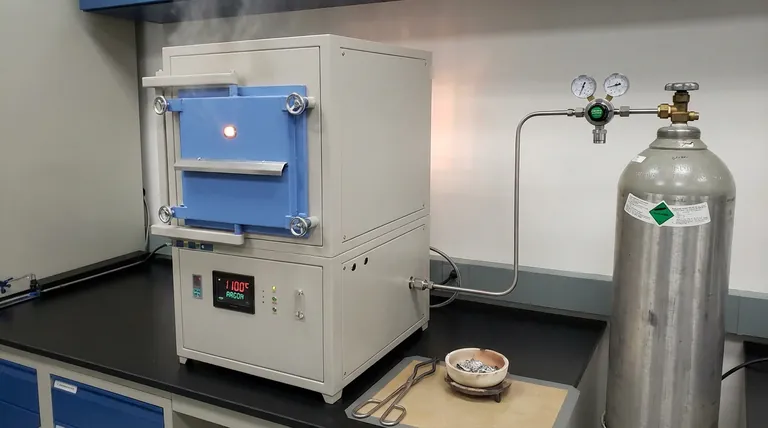
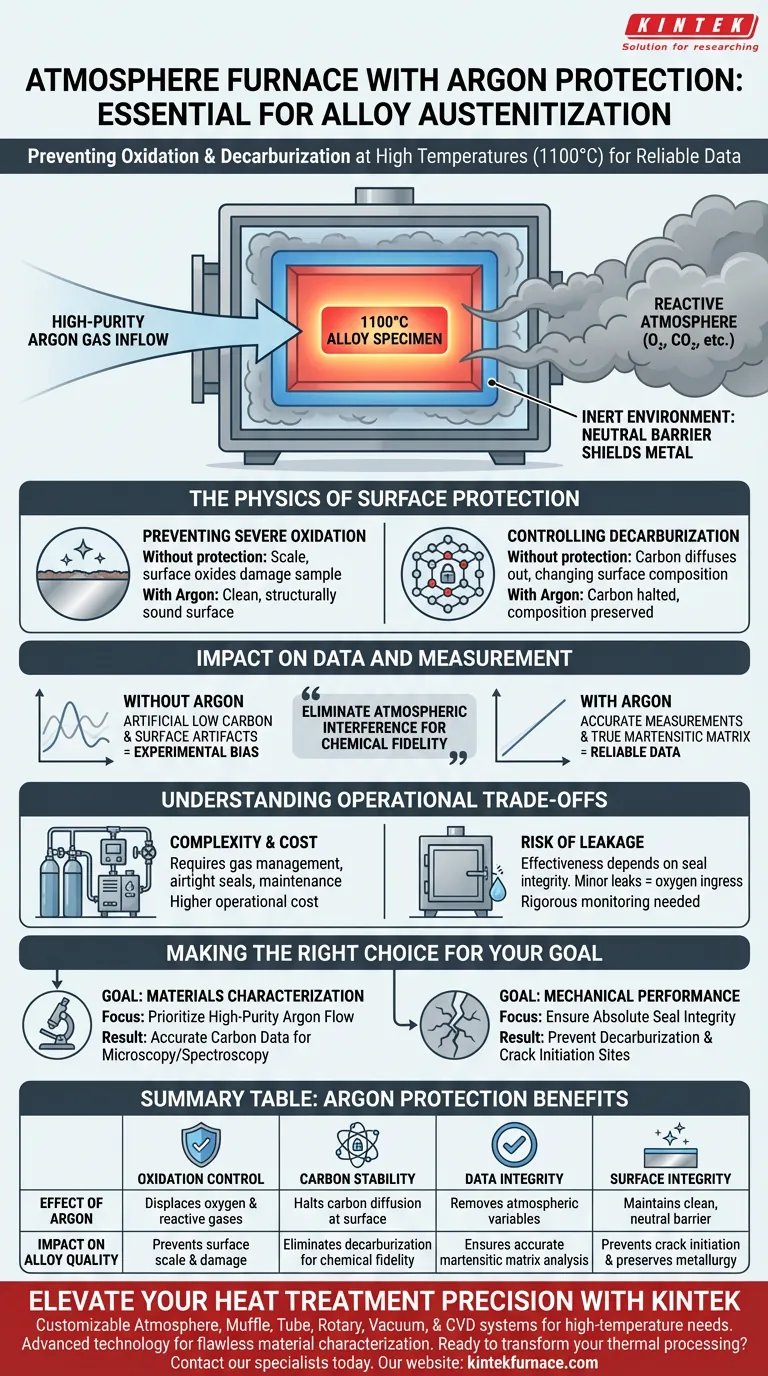
Using an atmosphere furnace supplied with high-purity argon gas is essential to create an inert environment that shields the alloy from reaction with the surrounding air. During high-temperature austenitization at 1100°C, this protection directly prevents severe oxidation and decarburization, preserving the surface integrity of the specimen.
The primary value of this setup is the preservation of chemical fidelity. By eliminating atmospheric interference, you ensure that measurements of local carbon content reflect the true state of the martensitic matrix rather than surface artifacts created by high-heat exposure.
The Physics of Surface Protection
Creating an Inert Environment
At elevated temperatures such as 1100°C, alloy surfaces become highly reactive.
The introduction of high-purity argon gas displaces oxygen and other reactive atmospheric elements within the furnace chamber.
This creates a neutral barrier that physically prevents the atmosphere from interacting with the hot metal.
Preventing Severe Oxidation
Without protection, the high heat promotes rapid oxidation on the specimen's exterior.
This results in the formation of scale or surface oxides that damage the sample.
Argon protection mitigates this, ensuring the material remains clean and structurally sound.
Controlling Decarburization
High temperatures can cause carbon atoms to diffuse out of the alloy’s surface layers, a process known as decarburization.
This loss changes the chemical composition of the surface, making it distinct from the core material.
An inert argon atmosphere effectively halts this diffusion process, locking the carbon within the lattice.
Impact on Data and Measurement
Ensuring Accurate Carbon Analysis
To understand the properties of the martensitic matrix, you must measure the local carbon content precisely.
If the surface has suffered from decarburization, your measurements will show artificially low carbon levels.
Argon protection ensures that the surface composition remains representative of the bulk material.
Eliminating Experimental Bias
Scientific validity relies on minimizing external variables.
Allowing oxidation or decarburization introduces "noise" into your data, creating experimental bias.
By controlling the atmosphere, you isolate the variable of interest—the alloy's response to heat—ensuring your data is reliable.
Understanding the Operational Trade-offs
Equipment Complexity and Cost
While atmosphere furnaces provide superior protection, they require more complex infrastructure than standard box furnaces.
You must manage gas flow rates, ensure high-purity argon supplies, and maintain airtight seals.
This increases the operational cost and the technical maintenance required for the heat treatment process.
The Risk of Leakage
The effectiveness of this method is entirely dependent on the integrity of the furnace seal.
Even a minor leak can allow oxygen ingress, rendering the argon protection ineffective at these high temperatures.
Rigorous monitoring of the furnace atmosphere is required to prevent "invisible" contamination of the results.
Making the Right Choice for Your Goal
To maximize the quality of your heat treatment results, align your furnace settings with your specific objectives:
- If your primary focus is Materials Characterization: Prioritize high-purity argon flow to prevent surface chemistry changes, ensuring that subsequent microscopy or spectroscopy yields accurate carbon data.
- If your primary focus is Mechanical Performance: Ensure the seal integrity is absolute, as even minor surface decarburization can act as a crack initiation site during stress testing.
By strictly controlling the atmosphere, you transform a chaotic high-heat environment into a precise laboratory tool.
Summary Table:
| Feature | Effect of Argon Protection | Impact on Alloy Quality |
|---|---|---|
| Oxidation Control | Displaces oxygen and reactive gases | Prevents surface scale and specimen damage |
| Carbon Stability | Halts carbon diffusion at the surface | Eliminates decarburization for chemical fidelity |
| Data Integrity | Removes atmospheric variables | Ensures accurate measurements of martensitic matrix |
| Surface Integrity | Maintains clean, neutral barrier | Prevents crack initiation and preserves metallurgy |
Elevate Your Heat Treatment Precision with KINTEK
Don't let oxidation or decarburization compromise your research results. Backed by expert R&D and manufacturing, KINTEK offers high-performance Atmosphere, Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique high-temperature laboratory needs. Our advanced furnace technology ensures the inert environment required for flawless material characterization and mechanical testing.
Ready to transform your thermal processing? Contact our specialists today to find the perfect customized furnace solution for your lab!
Visual Guide
References
- H. SCHAEFER, Jonathan Lentz. Phase Analysis and Measurement of Local Carbon Contents in Hypoeutectic Alloys in the System Fe-C-B-Cr-W. DOI: 10.1007/s00501-024-01436-w
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the primary purpose of using a small controlled electric furnace? Optimize Black Liquor Pyrolysis for Research
- What is a retort furnace and what is its primary purpose? Master Controlled Heat Treatment for Your Materials
- What is a retort furnace and its primary function? Achieve High-Purity Thermal Processing in Sealed Environments
- What are the advantages of a hydrogen reducing atmosphere for stainless steel MIM parts? Achieve Superior Integrity
- What are the two main types of atmosphere furnaces based on design? Choose the Right Furnace for Your Lab
- What is the role of a laboratory annealing furnace in memristor fabrication? Enhance Interface & Stability
- What are the design configurations of retort furnaces? Optimize Your Thermal Processing with the Right Setup
- What is the difference between a vacuum furnace and an atmospheric furnace? Choosing the Right Thermal Process



















