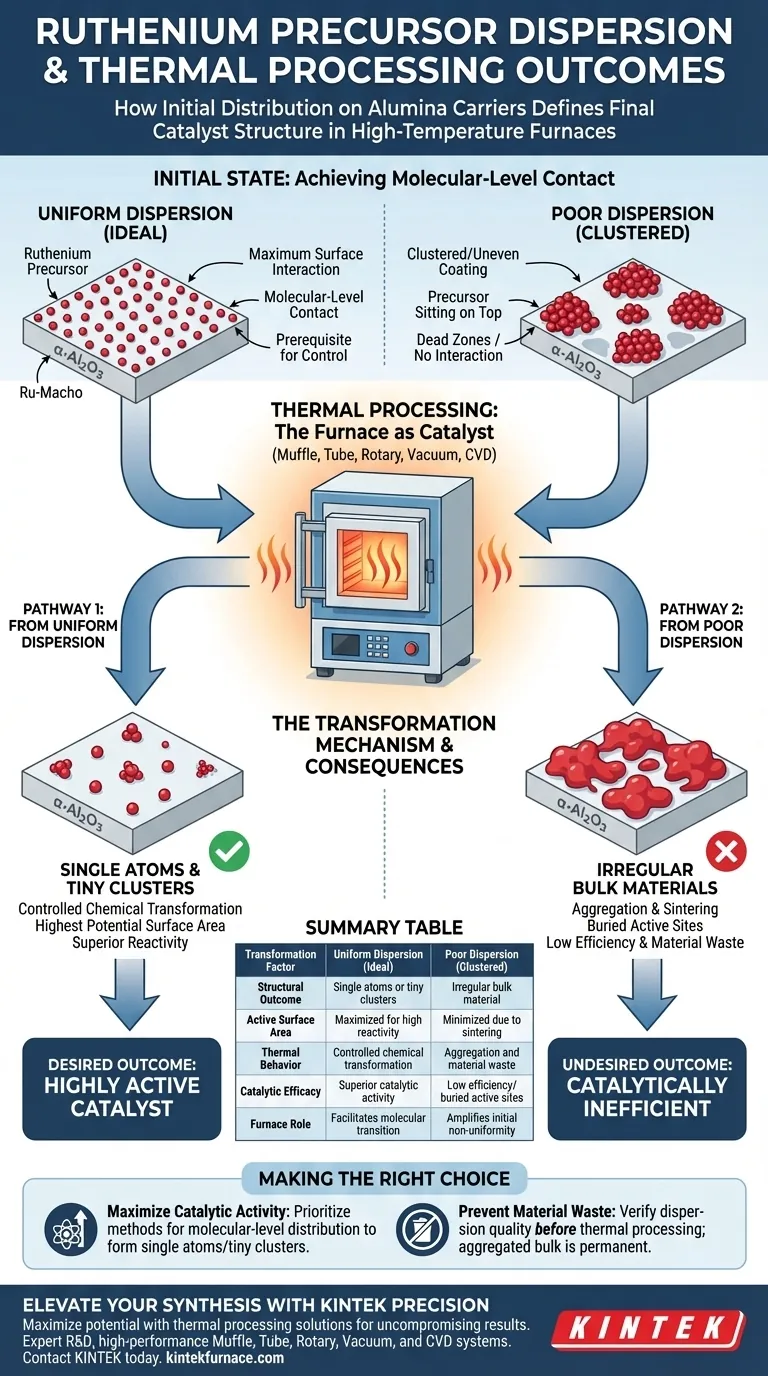
The dispersion quality of ruthenium precursors on alumina carriers is the defining variable that determines the structural integrity of your final catalytic material. When precursors like Ru-Macho are uniformly distributed, thermal processing in a high-temperature lab furnace successfully yields highly dispersed species, such as single atoms or tiny clusters. Conversely, poor dispersion leads to the formation of irregular bulk materials, significantly reducing the potential efficacy of the ruthenium.
The success of thermal processing is predetermined by the initial molecular-level contact between the active ruthenium components and the carrier surface; without this contact, the furnace promotes aggregation rather than the formation of discrete, active species.

The Mechanism of Precursor Transformation
Achieving Molecular-Level Contact
For a high-temperature lab furnace to function effectively, the starting material must have maximum surface interaction.
You must ensure that the ruthenium precursor is not merely sitting on top of the alpha-alumina (α-Al2O3) carrier, but is in contact at a molecular level.
This intimate contact is the prerequisite for controlling how the material behaves once heat is applied.
From Precursor to Active Species
The goal of thermal processing is to transform the precursor chemically without destroying its physical distribution.
When the dispersion is uniform, the thermal energy converts the precursor into highly specific forms, such as single atoms or nanometric clusters.
These forms represent the highest potential surface area and reactivity for the ruthenium.
The Consequence of Poor Dispersion
Formation of Irregular Bulk Materials
If the precursor is clustered or unevenly coated before entering the furnace, the high temperatures will cause the material to sinter together.
Instead of distinct, highly active atoms, you are left with irregular bulk ruthenium.
This result essentially wastes the potential of the precious metal by burying active sites inside larger, less reactive masses.
The Limits of Thermal Processing
It is critical to understand that the lab furnace cannot correct issues regarding initial uniformity.
The furnace acts as a catalyst for transformation, but it amplifies the state of the material as it enters the chamber.
Therefore, the thermal process is only as effective as the quality of the precursor's initial dispersion.
Understanding the Trade-offs
Process Preparation vs. Throughput
Achieving uniform dispersion often requires more time-intensive preparation steps before the material ever reaches the furnace.
Rushing the application of the precursor to the carrier to increase throughput will almost invariably result in lower-quality bulk material.
Sensitivity of Alpha-Alumina Carriers
Alpha-alumina is a robust carrier, but its effectiveness relies on accessible surface area.
Overloading the carrier or failing to spread the precursor thinly results in "dead zones" where the ruthenium cannot interact with the support.
This leads to a final product that is structurally weak and catalytically inefficient.
Making the Right Choice for Your Goal
To maximize the utility of your high-temperature lab furnace, you must align your preparation methods with your desired material properties.
- If your primary focus is maximizing catalytic activity: Prioritize methods that guarantee molecular-level distribution of the Ru-Macho precursor to ensure the formation of single atoms or tiny clusters.
- If your primary focus is preventing material waste: Verify the dispersion quality on the α-Al2O3 carrier before thermal processing, as aggregated bulk material cannot be easily redistributed once formed.
The furnace supplies the energy for transformation, but the quality of dispersion dictates the architecture of the result.
Summary Table:
| Transformation Factor | Uniform Dispersion (Ideal) | Poor Dispersion (Clustered) |
|---|---|---|
| Structural Outcome | Single atoms or tiny clusters | Irregular bulk material |
| Active Surface Area | Maximized for high reactivity | Minimized due to sintering |
| Thermal Behavior | Controlled chemical transformation | Aggregation and material waste |
| Catalytic Efficacy | Superior catalytic activity | Low efficiency/buried active sites |
| Furnace Role | Facilitates molecular transition | Amplifies initial non-uniformity |
Elevate Your Material Synthesis with KINTEK Precision
Maximize the potential of your ruthenium catalysts with thermal processing solutions that deliver uncompromising results. At KINTEK, we understand that the architecture of your final material depends on precise thermal control. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your specific research needs.
Whether you are developing single-atom catalysts or advanced nanometric clusters, our furnaces provide the uniform heating and stability required for sensitive precursors on alpha-alumina carriers. Don't let poor thermal control waste your precious metals.
Contact KINTEK today to discuss your custom furnace needs and ensure your lab achieves the highest level of catalytic efficiency.
Visual Guide
References
- DeSheng Su, Liang Chen. Efficient amine-assisted CO2 hydrogenation to methanol co-catalyzed by metallic and oxidized sites within ruthenium clusters. DOI: 10.1038/s41467-025-55837-7
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the purpose of using a PID controller to drive a heating furnace? Master Thermal Kinetics Precision
- How does the optical clarity of quartz tubes benefit laboratory processes? Enhance Control and Accuracy in High-Temp Experiments
- Why Is a High-Precision Heating and Stirring Platform Necessary for ZnO Sol-Gel Synthesis? Achieve Perfect Nanoparticles
- Why is the precise regulation of oxygen ratios via mass flow controllers critical for MCTV catalyst yield?
- Why is a high-purity alumina crucible used for cored wire experiments? Ensure Zero-Contamination Heat Transfer
- What maintenance is required after using the alumina furnace tube? Ensure Longevity and Purity in Your Lab
- What is the significance of quartz vacuum sealing technology in Dy4T1-xGa12 production? Ensure High-Purity Synthesis
- Why are graphite crucible furnaces used in vacuum or protective atmosphere environments? Prevent Oxidation and Ensure Purity



















