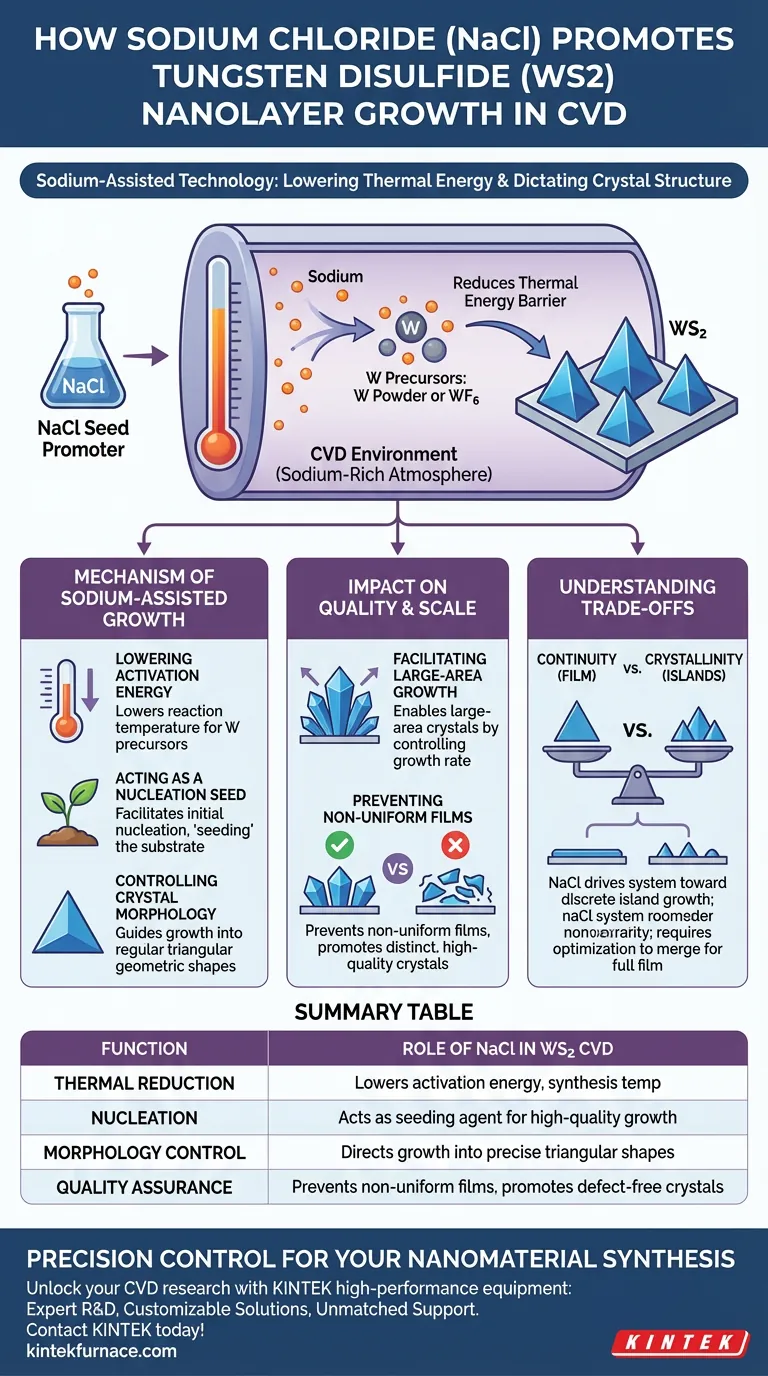
Sodium chloride (NaCl) functions as a critical seed promoter in the chemical vapor deposition (CVD) of tungsten disulfide (WS2) nanolayers. Through a process known as sodium-assisted technology, it significantly reduces the thermal energy required to react tungsten precursors while simultaneously dictating the geometric structure of the final crystal.
Core Takeaway: NaCl is not merely an additive; it modifies the growth environment to favor large, discrete, and high-quality crystals over messy, non-uniform films. It acts as a catalyst that lowers reaction temperatures and strictly controls the morphology of the resulting nanolayers.
The Mechanism of Sodium-Assisted Growth
Lowering the Activation Energy
The primary chemical function of NaCl in this process is thermal reduction. It lowers the reaction temperature required for tungsten precursors.
Whether you are using metal tungsten powder or tungsten hexafluoride (WF6), the presence of sodium reduces the energy barrier. This allows the synthesis to proceed efficiently at temperatures that would otherwise be insufficient for standard CVD.
Acting as a Nucleation Seed
NaCl acts directly as a seed promoter.
In the CVD environment, the salt creates a sodium-rich atmosphere. This facilitates the initial nucleation steps necessary to begin crystal growth, effectively "seeding" the substrate for the deposition of WS2.
Controlling Crystal Morphology
Beyond starting the reaction, NaCl governs the shape of the resulting material.
It guides the growth into regular triangular geometric shapes. This geometric control is essential for applications requiring precise crystallographic orientation and prevents the formation of irregular, amorphous structures.
Impact on Quality and Scale
Facilitating Large-Area Growth
The addition of NaCl enables the synthesis of large-area crystals.
By controlling the nucleation and growth rate, the sodium atmosphere allows individual crystals to expand laterally. This results in significant surface area coverage while maintaining structural integrity.
Preventing Non-Uniform Films
A common challenge in CVD is the unintentional formation of continuous films that suffer from variable quality.
NaCl specifically prevents the formation of these non-uniform continuous films. Instead, it promotes the growth of distinct, high-quality crystals with controllable sizes, ensuring that the resulting material is uniform and defect-free.
Understanding the Trade-offs
Continuity vs. Crystallinity
While NaCl improves individual crystal quality, it is designed to prevent continuous film formation in favor of discrete shapes.
If your specific application requires a fully continuous, unbroken sheet of WS2 rather than distinct triangular domains, you must recognize that NaCl drives the system toward discrete island growth. Achieving a full film would require optimizing the process to merge these high-quality triangles without introducing grain boundaries.
Making the Right Choice for Your Project
To leverage sodium-assisted CVD effectively, align the method with your specific limitations and goals:
- If your primary focus is crystal quality: Use NaCl to ensure the growth of regular, triangular geometric shapes rather than irregular structures.
- If your primary focus is thermal constraints: Rely on NaCl to significantly lower the reaction temperature of precursors like WF6 or tungsten powder.
- If your primary focus is uniformity: Utilize this method to prevent the formation of non-uniform continuous films and ensure controllable crystal sizes.
By treating NaCl as a functional catalyst rather than a passive ingredient, you gain precise control over both the thermodynamics and morphology of your WS2 nanolayers.
Summary Table:
| Function | Role of NaCl in WS2 CVD |
|---|---|
| Thermal Reduction | Lowers activation energy and synthesis temperatures for tungsten precursors |
| Nucleation | Acts as a seeding agent to initiate high-quality crystal growth |
| Morphology Control | Directs growth into precise, discrete triangular geometric shapes |
| Quality Assurance | Prevents non-uniform films by promoting large-area, defect-free crystals |
Precision Control for Your Nanomaterial Synthesis
Unlock the full potential of your CVD research with high-performance equipment from KINTEK. Whether you are implementing sodium-assisted growth for WS2 or exploring complex thin-film depositions, our systems provide the thermal stability and atmospheric control required for success.
Why choose KINTEK?
- Expert R&D & Manufacturing: Precision-engineered Muffle, Tube, Rotary, Vacuum, and CVD systems.
- Customizable Solutions: High-temperature furnaces tailored to your unique experimental parameters.
- Unmatched Support: Technical guidance to help you achieve uniform, high-quality crystal growth.
Contact KINTEK today to optimize your lab's high-temperature processes!
Visual Guide
References
- O. Ozturk, Emre Gür. Layered Transition Metal Sulfides for Supercapacitor Applications. DOI: 10.1002/celc.202300575
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What are the main types of thin film deposition processes? Compare PVD vs. CVD for Your Lab
- What materials are used in the hot zone of CVD furnaces? Optimize for Purity, Cost, and Performance
- What are the temperature control requirements for HCVD furnaces? Achieve Precise Multi-Zone Thermal Management
- What non-metal elements are commonly deposited using CVD? Discover Silicon and Carbon Applications
- Why is CVT preferred over solid-phase reaction for Janus RhSeCl? Key Advantages in Crystal Growth
- What role does a trace moisture injection device play in Super-growth CVD? Unlock High-Purity CNT Synthesis
- Why are diffusion or turbomolecular pumps necessary for PVD? Ensure Pure, High-Hardness Thin Film Coatings
- What is Chemical Vapor Deposition (CVD) and what is its primary function? | Enhance Materials with Precision Coatings














