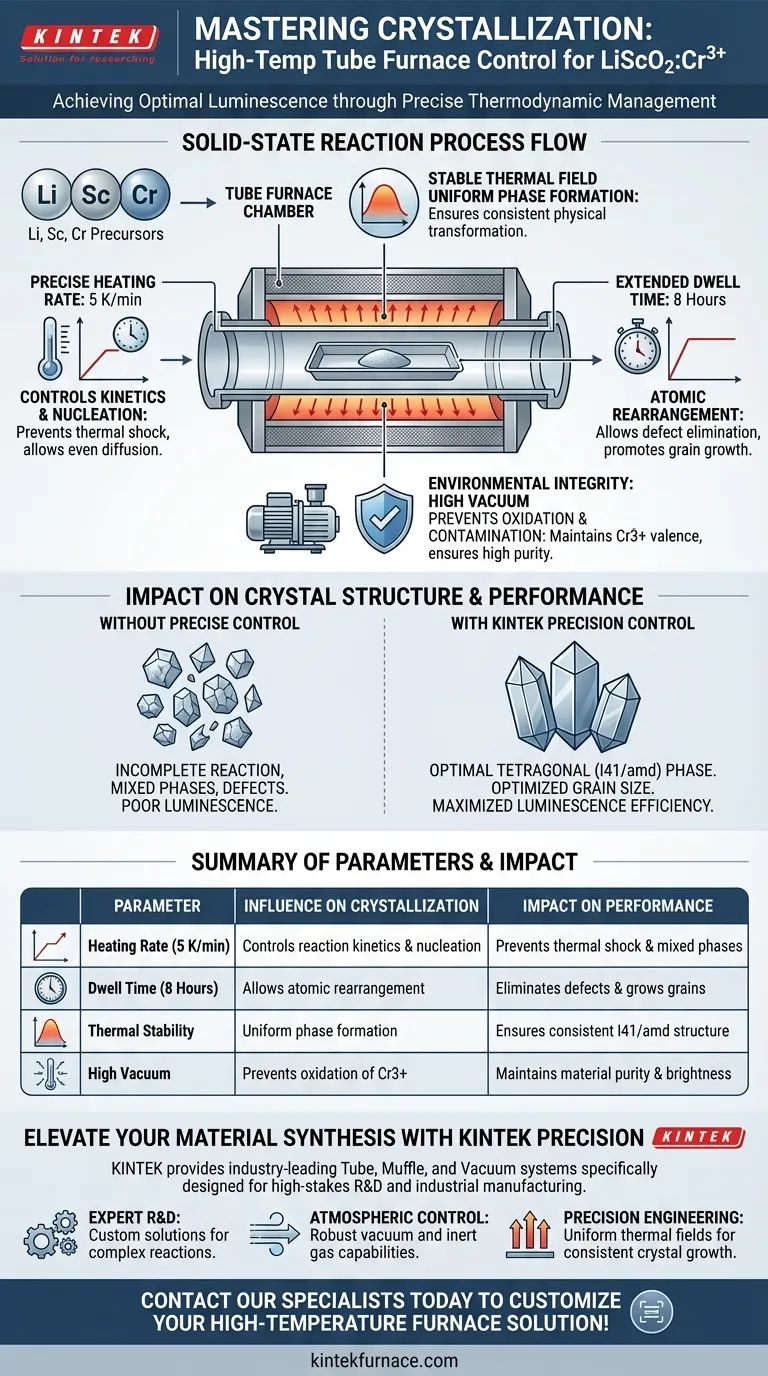
Industrial high-temperature tube furnaces govern crystallization quality by enforcing strict thermodynamic parameters during the solid-state reaction of LiScO2:Cr3+. By maintaining precise heating rates, such as 5 K/min, and holding high temperatures for extended periods, typically around 8 hours, these systems ensure the formation of the optimal tetragonal crystal phase. This process allows for the specific adjustment of grain size, which is the primary driver of the phosphor’s final luminescence efficiency.
The key to high-quality LiScO2:Cr3+ synthesis is not merely reaching a temperature peak, but managing the stability of the thermal field to dictate phase formation and grain growth.

The Role of Thermodynamic Precision
To achieve a high-performance phosphor, you must move beyond simple heating and focus on the kinetics of the reaction.
Establishing a Stable Thermal Field
The primary function of the tube furnace is to provide a uniform and stable thermal environment.
Inconsistencies in temperature can lead to incomplete reactions or mixed phases. A stable thermal field ensures that every part of the sample undergoes the same physical transformation simultaneously.
Controlling Reaction Kinetics
The heating rate is a critical variable in the solid-state reaction.
Setting a specific rate, such as 5 K/min, prevents thermal shock and allows the reactants to diffuse evenly. This controlled ramp-up is essential for initiating the nucleation process correctly.
The Importance of Dwell Time
Reaching the target temperature is only the beginning; maintaining it is where the crystallization matures.
Extended isothermal periods, such as 8 hours, provide the necessary time for the atoms to rearrange into the desired structure. This "soak time" is non-negotiable for eliminating structural defects.
Impact on Crystal Structure and Performance
The physical settings of the furnace directly translate to the atomic structure of the material.
Achieving the Correct Phase
The goal of the solid-state reaction is to stabilize the material into a specific tetragonal crystal phase.
This phase is identified as the I41/amd space group. Without the precise thermal history provided by the furnace, the material may settle into a less stable or non-luminescent phase.
Optimizing Grain Size
The duration and temperature of the annealing process directly control the growth of crystal grains.
Larger, well-formed grains generally exhibit fewer surface defects. Optimizing these annealing parameters is the most effective way to maximize the luminescence efficiency of the phosphor.
Environmental Integrity and Purity
While heat drives the reaction, the atmosphere within the furnace protects the material's integrity.
Protecting Against Oxidation
High-temperature environments can aggressively accelerate oxidation.
Operating under a high vacuum prevents oxygen from interacting with the sample. This is vital for maintaining the valence state of the dopants, specifically Cr3+.
Preventing Contamination
Reaction with surrounding gases can introduce impurities that quench luminescence.
A vacuum environment isolates the LiScO2:Cr3+ from external contaminants. This ensures that the final product retains high purity, which is critical for consistent optical performance.
Understanding the Trade-offs
Achieving high crystallization quality requires balancing precision with efficiency.
Process Time vs. Throughput
The requirement for slow heating rates (5 K/min) and long dwell times (8 hours) significantly lengthens the production cycle.
High-quality crystallization is inherently slow. Attempting to rush this process to increase throughput often results in smaller grain sizes and inferior luminescence.
Complexity of Atmosphere Control
Maintaining a high vacuum adds a layer of operational complexity and cost.
While vacuum protects the sample, it requires rigorous maintenance of seals and pumps. Any leak in the system during high-temperature treatment can compromise the entire batch.
Making the Right Choice for Your Goal
When configuring your tube furnace for LiScO2:Cr3+ synthesis, align your parameters with your specific performance metrics.
- If your primary focus is Luminescence Efficiency: Prioritize extended isothermal holds (e.g., 8 hours) to maximize grain growth and reduce defects in the I41/amd phase.
- If your primary focus is Material Purity: Ensure your system can maintain a robust high vacuum to eliminate oxidation and gas-phase contamination.
Ultimately, superior crystallization is the result of patience and precision, where the stability of the thermal field determines the brilliance of the final product.
Summary Table:
| Parameter | Influence on Crystallization | Impact on Performance |
|---|---|---|
| Heating Rate (5 K/min) | Controls reaction kinetics & nucleation | Prevents thermal shock & mixed phases |
| Dwell Time (8 Hours) | Allows atomic rearrangement | Eliminates defects & grows grains |
| Thermal Stability | Uniform phase formation | Ensures consistent I41/amd structure |
| High Vacuum | Prevents oxidation of Cr3+ | Maintains material purity & brightness |
Elevate Your Material Synthesis with KINTEK Precision
Achieving the perfect tetragonal phase in LiScO2:Cr3+ requires absolute control over every thermal variable. KINTEK provides industry-leading Tube, Muffle, and Vacuum systems specifically designed for high-stakes R&D and industrial manufacturing. Our furnaces offer the stability and atmospheric integrity necessary to maximize grain size and luminescence efficiency.
Why choose KINTEK?
- Expert R&D: Custom solutions for complex solid-state reactions.
- Atmospheric Control: Robust vacuum and inert gas capabilities to prevent contamination.
- Precision Engineering: Uniform thermal fields for consistent crystal growth.
Contact our specialists today to customize your high-temperature furnace solution!
Visual Guide
References
- Leoni Frehmeyer, Thomas Jüstel. On the optimisation of the broadband NIR emitter LiScO2:Cr3+. DOI: 10.6001/chemija.2025.36.2.5
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What advanced control features do modern tube furnaces have? Precision Temperature, Atmosphere, and Data Control
- What functions does a support frame provide in tube furnace modernization? Gain Stability and Experimental Flexibility
- How does a fast Joule-heating device differ from a tubular furnace? Kinetic vs. Thermodynamic Control
- How does the positioning of a quartz tube in a vertical tube furnace contribute to the stability of the synthesis reaction?
- What role does a vacuum tube furnace play in AlCrSiWN coating annealing? Enhance Stability and Hardness
- What optional features are available for tube furnaces? Enhance Your Materials Processing with Precision Control
- How does an alumina-lined vertical tube furnace provide a stable environment for corrosion experiments? Get Expert Data
- What industries commonly use tube furnaces? Essential for High-Tech Materials and Electronics



















