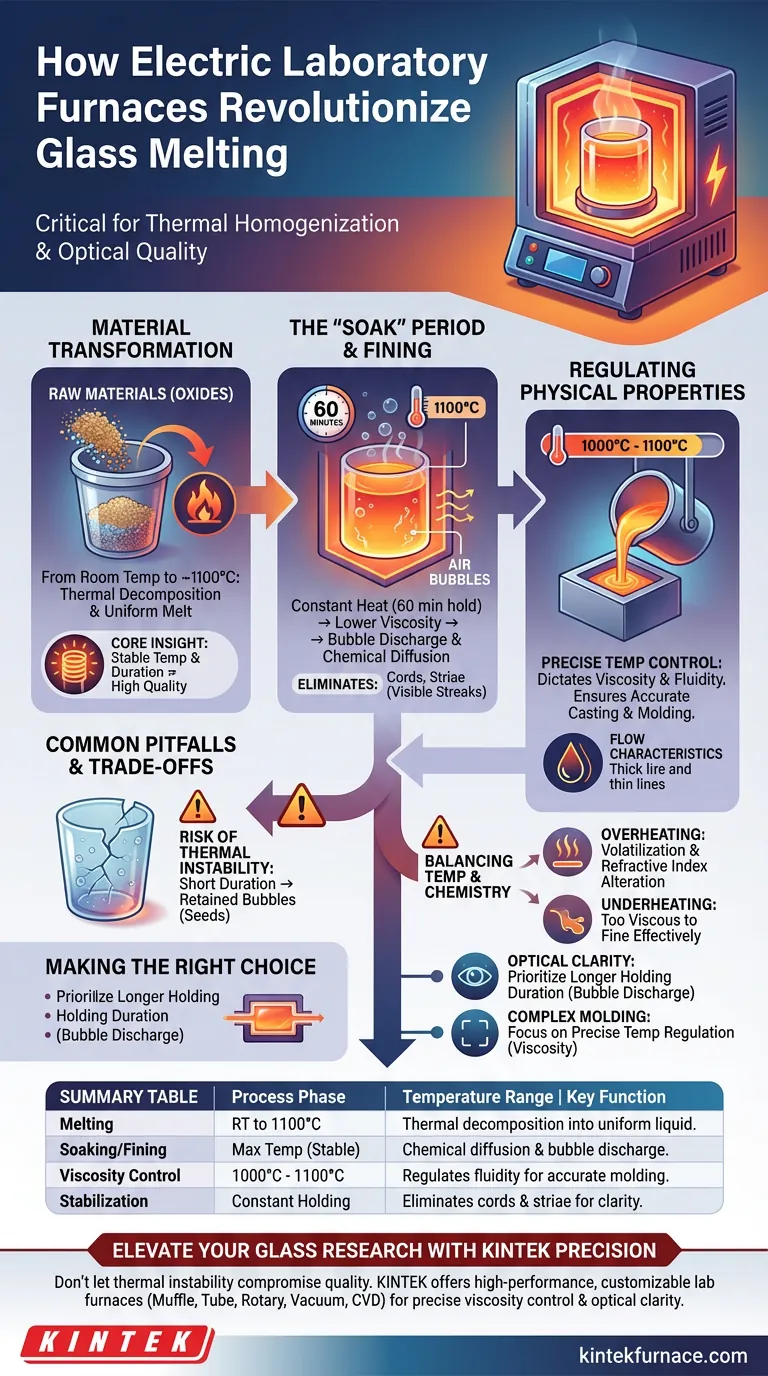
The electric laboratory furnace serves as the critical instrument for thermal homogenization in the glass manufacturing process. It provides a precisely controlled environment to raise raw materials from room temperature to approximately 1100°C, ensuring that oxide powders decompose and melt completely into a uniform liquid state.
Core Insight Simply reaching a melting point is not enough for high-quality glass. The furnace’s true value lies in its ability to maintain a stable temperature for a specific duration, allowing for the diffusion of chemical components and the escape of internal air bubbles—steps that are non-negotiable for producing optical-grade material.
The Mechanics of Material Transformation
Achieving a Uniform Melt
The primary function of the furnace is to facilitate the physicochemical reactions of raw materials. As the furnace heats from room temperature to 1100°C, it triggers the thermal decomposition of oxide powders.
This intense, controlled heat drives the transition from a solid, granular mixture into a cohesive, uniform liquid. Without this specific thermal consistency, the glass would contain unmelted batch materials, rendering it useless.
The Role of the "Soak" Period
Once the target temperature is reached, the furnace plays a crucial role in stabilization. The process often requires maintaining the maximum temperature for a set duration, such as 60 minutes.
During this holding period, the constant heat lowers the glass viscosity enough to allow internal air bubbles to rise and discharge. Simultaneously, it allows chemical components to diffuse evenly throughout the mixture, eliminating cords or striae (visible streaks) in the final product.
Regulating Physical Properties
Controlling Viscosity and Fluidity
The electric furnace acts as a regulator for the glass melt's flow characteristics. The precise temperature control—often within a tight range around 1000°C to 1100°C—directly dictates the viscosity of the molten glass.
By managing the heat input, the furnace ensures the fluid has the correct "thickness" for processing. This directly impacts the quality of subsequent casting and molding steps, ensuring the glass fills molds accurately without premature cooling.
Common Pitfalls and Trade-offs
The Risk of Thermal Instability
While electric furnaces provide high precision, failing to maintain the specific holding time or temperature profile leads to defects. If the duration is too short, the glass will retain bubbles (seeds).
Balancing Temperature and Chemistry
There is a delicate balance between temperature and chemical composition. Overheating can lead to the volatilization of certain volatile components, altering the glass's final refractive index. Conversely, underheating results in a mixture that is too viscous to fine (remove bubbles) effectively.
Making the Right Choice for Your Goal
To maximize the utility of an electric laboratory furnace, align your thermal profile with your specific output requirements:
- If your primary focus is Optical Clarity: Prioritize a longer holding duration at peak temperature to ensure maximum bubble discharge and chemical diffusion.
- If your primary focus is Complex Molding: Focus on precise temperature regulation to maintain the exact viscosity and fluidity required to fill intricate mold details.
Precision heating is the bridge between raw chemical potential and a flawless, optical-quality final product.
Summary Table:
| Process Phase | Temperature Range | Key Function in Glass Production |
|---|---|---|
| Melting | RT to 1100°C | Thermal decomposition of oxides into a uniform liquid state. |
| Soaking/Fining | Max Temp (Stable) | Facilitates chemical diffusion and internal bubble discharge. |
| Viscosity Control | 1000°C - 1100°C | Regulates fluidity for accurate molding and casting. |
| Stabilization | Constant Holding | Eliminates cords and striae for optical-grade clarity. |
Elevate Your Glass Research with KINTEK Precision
Don't let thermal instability compromise your glass quality. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable for your specific glass melting and thermal processing needs. Whether you require precise viscosity control or superior optical clarity, our lab high-temp furnaces provide the stability your materials demand.
Ready to refine your results? Contact our experts today to find the perfect furnace solution for your laboratory.
Visual Guide
References
- Fathy Abdel-Wahab, Heba Abdelmaksoud. Investigation of oxygen defects in chromium-doped borosilicate glass co-doped with alkali metal (Na2O) and transition metal (ZnO) for photonic applications. DOI: 10.1007/s00339-024-08114-1
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- In which industries are muffle furnaces commonly used? Essential for Clean High-Temp Processing
- How does a high-temperature box furnace contribute to the accuracy of oxidation kinetics experiments? Achieve Precision
- What materials are used in the construction of muffle furnaces? Key Components for High-Temp Performance
- What is the primary function of an industrial box furnace? Master 60Si2CrV Spring Steel Heat Treatment
- What is the function of a high-temperature box-type resistance furnace in rGO synthesis? Optimize Your Carbonization
- Why is a high-temperature muffle furnace required for ash determination? Ensure Precise Edible Mushroom Analysis
- What makes box furnaces suitable for demanding applications? Engineered for Precision and Durability in High-Stakes Processes
- How does a high-precision furnace enhance EIS testing for niobium-doped titanium dioxide? Achieve Accurate Material Data



















