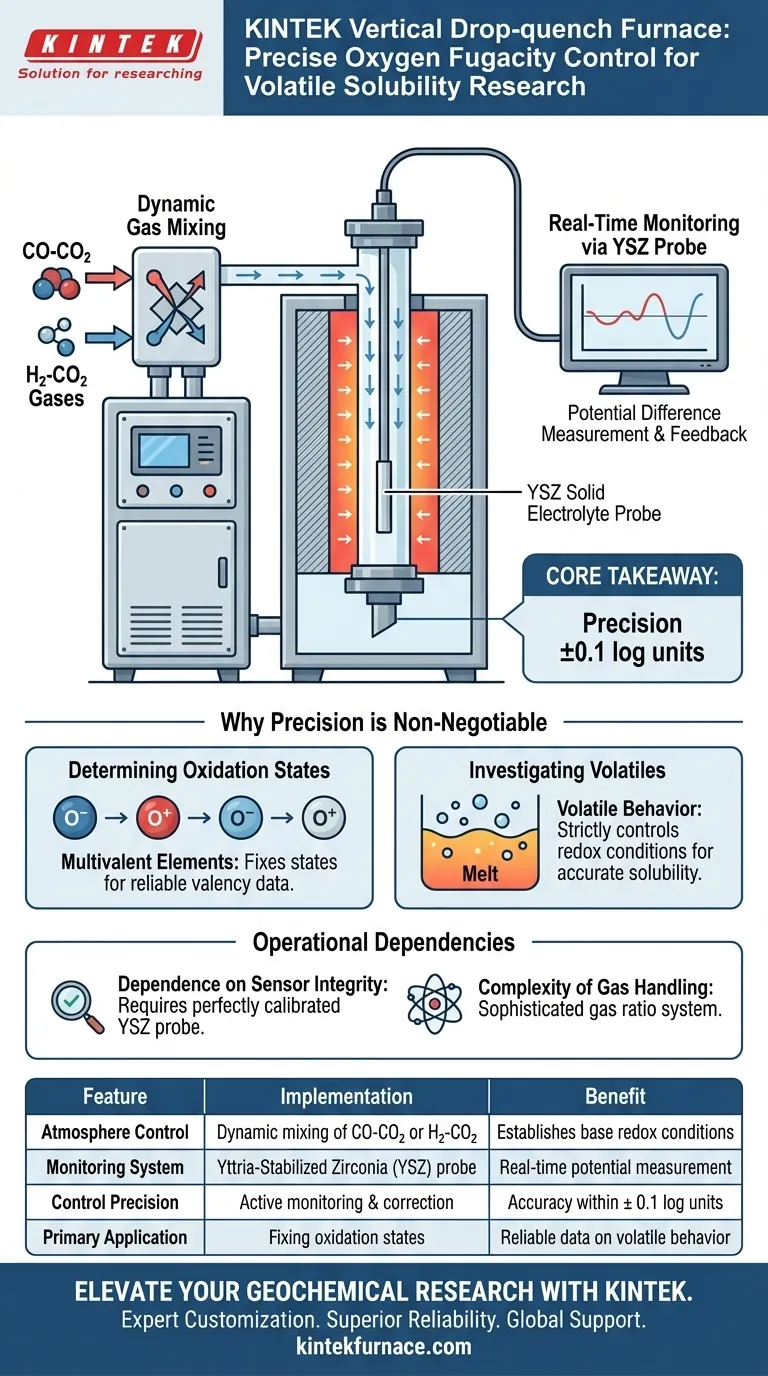
A Vertical Drop-quench Furnace achieves precise oxygen fugacity control by dynamically introducing specific ratios of mixed gases, such as CO-CO$_2$ or H$_2$-CO$_2$, into the reaction tube. To ensure accuracy, the system relies on a Yttria-Stabilized Zirconia (YSZ) solid electrolyte probe, which measures the potential difference in real-time to maintain the environment within strict limits.
Core Takeaway The combination of calibrated gas mixing and real-time electrolytic monitoring allows these furnaces to maintain oxygen fugacity within a precision of $\pm 0.1$ log units. This tight control is the foundation for accurately determining the oxidation states of multivalent elements and investigating volatile behavior in experimental melts.

The Mechanics of Atmosphere Control
To understand how the furnace achieves its precision, it is necessary to look at the interplay between the gas supply and the monitoring system.
Dynamic Gas Mixing
The furnace does not rely on a static environment. Instead, it introduces a continuous flow of mixed gases into the reaction tube.
The most common mixtures utilized are CO-CO$_2$ (Carbon Monoxide and Carbon Dioxide) or H$_2$-CO$_2$ (Hydrogen and Carbon Dioxide). By adjusting the ratio of these gases, the baseline oxygen fugacity is established.
Real-Time Monitoring via YSZ Probe
Gas flow alone is insufficient for high-precision research. The system is equipped with an oxygen probe based on a Yttria-Stabilized Zirconia (YSZ) solid electrolyte.
This probe acts as a real-time sensor. It measures the potential difference within the furnace, providing immediate feedback on the actual oxygen conditions.
Achieving High Precision
The integration of the gas mixtures with the YSZ probe allows for a control precision of $\pm 0.1$ log units.
This specific tolerance level is not arbitrary; it represents the threshold required to replicate accurate geological conditions experimentally.
Why Precision is Non-Negotiable
The technical capability of the furnace serves a deeper scientific need: the isolation of chemical variables in the melt.
Determining Oxidation States
Many elements in geological melts are multivalent, meaning they can exist in multiple oxidation states depending on the environment.
Precise control of oxygen fugacity is essential to fix these states. Without the $\pm 0.1$ log unit precision, the resulting data regarding the valency of these elements would be unreliable.
Investigating Volatiles
Volatiles behave differently depending on the redox conditions of the melt.
To accurately determine how volatiles dissolve or exsolve, the experimental environment must strictly control the oxygen fugacity. Any fluctuation outside the control range could alter the behavior of the volatiles, leading to erroneous solubility data.
Understanding the Operational Dependencies
While this system offers high precision, it introduces specific dependencies that can be viewed as operational trade-offs or constraints.
Dependence on Sensor Integrity
The entire control loop relies heavily on the YSZ solid electrolyte probe.
Unlike simpler buffer techniques (which rely on chemical equilibrium of solids), this method requires the probe to be perfectly calibrated and functioning. If the probe's measurement of potential difference drifts or fails, the gas mixture may become inaccurate despite the flow settings remaining constant.
Complexity of Gas Handling
The requirement for CO-CO$_2$ or H$_2$-CO$_2$ mixtures necessitates a sophisticated gas handling system.
This adds a layer of complexity compared to inert atmosphere furnaces. The user must ensure the gas ratios are precise before they even enter the reaction tube to allow the YSZ probe to fine-tune the final environment.
Making the Right Choice for Your Research
When utilizing a Vertical Drop-quench Furnace, align your experimental setup with your specific data requirements.
- If your primary focus is Multivalent Elements: Ensure your gas ratios are stabilized to maintain the $\pm 0.1$ log unit precision required to distinguish between subtle changes in oxidation states.
- If your primary focus is Volatile Solubility: Prioritize the responsiveness of the YSZ probe to ensure the melt environment remains constant throughout the equilibration period.
Ultimately, the value of this apparatus lies in its ability to actively monitor and correct the reaction environment in real-time.
Summary Table:
| Feature | Implementation Mechanism | Benefit for Research |
|---|---|---|
| Atmosphere Control | Dynamic mixing of CO-CO2 or H2-CO2 gases | Establishes base redox conditions for solubility |
| Monitoring System | Yttria-Stabilized Zirconia (YSZ) probe | Real-time potential measurement and feedback |
| Control Precision | Active monitoring & correction loops | Accuracy within $\pm 0.1$ log units |
| Primary Application | Fixing oxidation states of multivalent elements | Reliable data on volatile behavior and valency |
Elevate Your Geochemical Research with KINTEK
Precise redox control is the difference between breakthrough data and erroneous results. At KINTEK, we understand the rigorous demands of volatile solubility and melt research. Backed by expert R&D and world-class manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, including specialized vertical furnaces customizable for your unique oxygen fugacity requirements.
Partner with KINTEK for:
- Expert Customization: Tailored furnace solutions for specific gas-mixing needs.
- Superior Reliability: Systems designed for high-stability and real-time monitoring.
- Global Support: Specialized engineering advice for your lab’s thermal processing challenges.
Contact KINTEK Today to Design Your Custom High-Temp System
Visual Guide
References
- Célia Dalou, Paolo A. Sossi. Review of experimental and analytical techniques to determine H, C, N, and S solubility and metal–silicate partitioning during planetary differentiation. DOI: 10.1186/s40645-024-00629-8
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What is the significance of using a vacuum diffusion annealing furnace for thermodynamic equilibrium studies in alloys?
- What are the advantages of using vacuum-based processing for Co3O4@CNT composites? Preserve 3D Architecture Today
- Why is HIP post-treatment required for ceramics? Achieve Zero Porosity and Maximum Optical Clarity
- What are some common industrial uses of vacuum furnaces? Enhance Material Quality and Performance
- What is the temperature of vacuum hardening? A Guide to Precise Heat Treatment
- How are vacuum brazing challenges overcome in furnace design? Master Precision and Purity for Strong Joints
- How do costs compare between low vacuum and high vacuum furnaces? Find the Best Fit for Your Budget and Needs
- What functions does a high-temperature sintering furnace perform in the preparation of porous magnesium oxide?



















