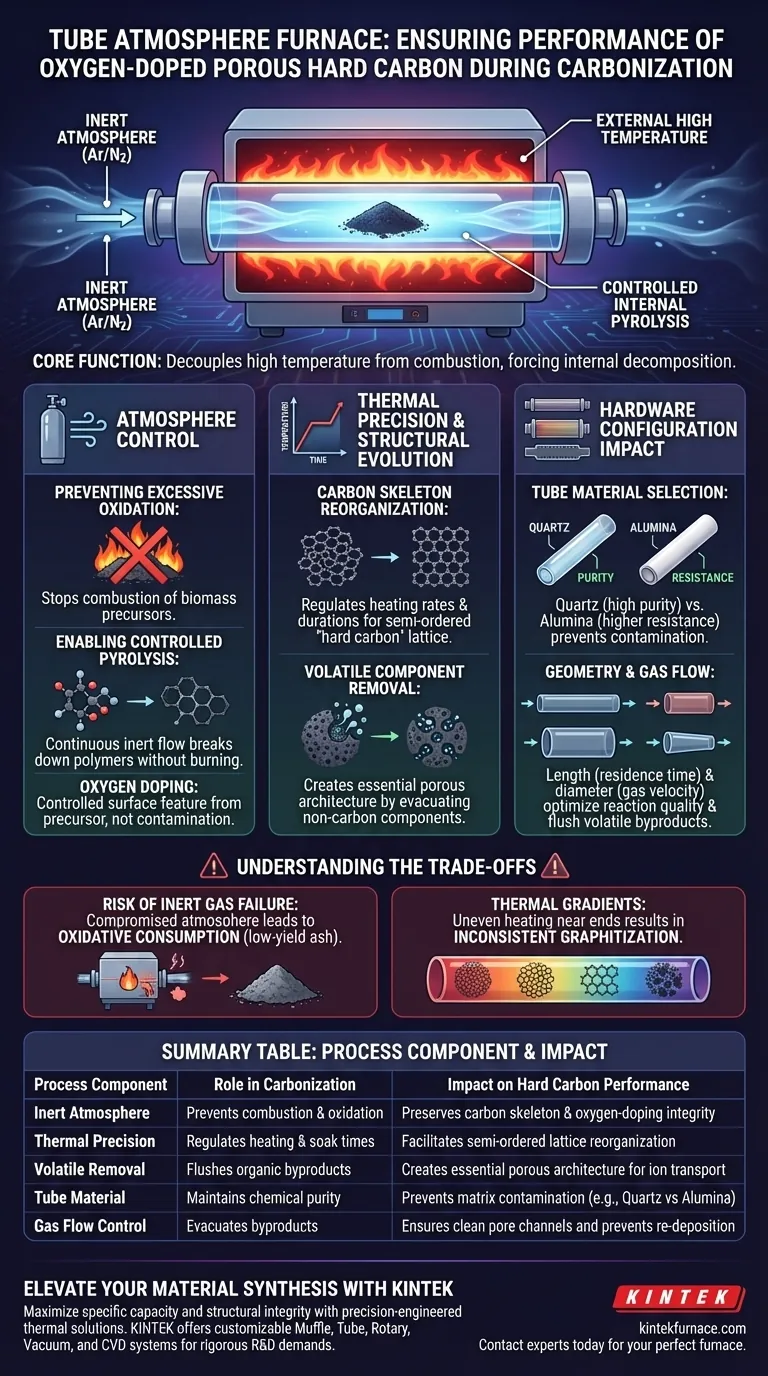
A tube atmosphere furnace safeguards the integrity of oxygen-doped porous hard carbon by isolating the biomass precursor in a strictly inert environment, usually argon or nitrogen, while applying precise thermal energy. This isolation prevents the material from burning to ash, allowing it to undergo controlled pyrolysis where volatile components are removed and the carbon skeleton is successfully reorganized for energy storage applications.
The core function of this equipment is to decouple high temperatures from combustion. By maintaining an oxygen-free atmosphere, the furnace forces the material to decompose internally rather than react with the air, ensuring the preservation of the carbon structure and the retention of specific capacity needed for potassium-ion batteries.

The Role of Atmosphere Control
Preventing Excessive Oxidation
The primary threat to carbonization is uncontrolled oxidation. Without a strict inert atmosphere, biomass precursors exposed to high temperatures would simply combust.
Enabling Controlled Pyrolysis
The furnace uses a continuous flow of inert gas (Argon or Nitrogen) to facilitate pyrolysis. This process breaks down complex organic polymers into simpler molecules without burning the carbon backbone.
This environment ensures that oxygen doping remains a controlled surface feature derived from the precursor (such as pre-oxidized bark), rather than the result of uncontrolled environmental contamination.
Thermal Precision and Structural Evolution
Reorganization of the Carbon Skeleton
The furnace allows for the precise regulation of heating rates and constant temperature durations. This control is critical for transforming the disordered structure of the raw biomass into a semi-ordered "hard carbon" lattice.
During this phase, the carbon layers realign. This reorganization creates the distinct microstructure required to host ions (like potassium) efficiently, leading to high specific capacity.
Removal of Volatile Components
As the temperature rises, the furnace ensures the efficient removal of non-carbon volatile components. This creates the necessary porous architecture within the material.
The evacuation of these volatiles generates the micropores and channels that define the material's surface area. This porosity is essential for electrolyte penetration and excellent rate performance in batteries.
The Impact of Hardware Configuration
Tube Material Selection
The choice of the furnace tube itself acts as a safeguard for process purity. Quartz tubes are often selected for these processes to ensure high purity and prevent contamination of the carbon matrix.
For processes requiring higher chemical resistance or thermal stability, alumina tubes may be utilized. This ensures the tube does not degrade and release impurities into the carbon during the high-heat soak.
Geometry and Gas Flow
The dimensions of the tube impact the reaction quality. A longer tube length can increase the residence time of the gas, ensuring the carbon is fully enveloped in the protective atmosphere throughout the reaction.
Conversely, the diameter effects gas velocity. Proper sizing ensures that volatile byproducts are flushed out effectively, preventing them from re-depositing on the carbon surface and clogging the newly formed pores.
Understanding the Trade-offs
The Risk of Inert Gas Failure
If the inert atmosphere is compromised—due to leaks or insufficient flow rates—the "hard carbon" will suffer from oxidative consumption. Instead of a conductive porous anode, you may end up with low-yield ash or a material with degraded structural integrity.
Thermal Gradients
While tube furnaces offer precise control, they can suffer from thermal gradients near the ends of the tube. If the sample is not positioned in the uniform temperature zone (usually the center), the carbonization will be uneven.
This uneven heating leads to inconsistent degrees of graphitization across the sample batch, resulting in unpredictable battery performance and varying capacity metrics.
Making the Right Choice for Your Goal
When configuring a tube atmosphere furnace for carbon synthesis, align your setup with your specific electrochemical targets:
- If your primary focus is maximizing specific capacity: Prioritize precise temperature stability to ensure complete skeleton reorganization without destroying the microstructure.
- If your primary focus is material purity: Select a quartz tube and ensure a high-purity inert gas source (like Argon) to eliminate trace contaminants.
- If your primary focus is pore structure development: Optimize the gas flow rate and tube length to control the evacuation speed of volatile components.
Success in carbonization is not just about heating a sample; it is about precisely managing the exclusion of oxygen to force the material to rebuild itself from the inside out.
Summary Table:
| Process Component | Role in Carbonization | Impact on Hard Carbon Performance |
|---|---|---|
| Inert Atmosphere | Prevents combustion & oxidation | Preserves carbon skeleton & oxygen-doping integrity |
| Thermal Precision | Regulates heating & soak times | Facilitates semi-ordered lattice reorganization |
| Volatile Removal | Flushes organic byproducts | Creates essential porous architecture for ion transport |
| Tube Material | Maintains chemical purity | Prevents matrix contamination (e.g., Quartz vs Alumina) |
| Gas Flow Control | Evacuates byproducts | Ensures clean pore channels and prevents re-deposition |
Elevate Your Material Synthesis with KINTEK
Maximize your specific capacity and structural integrity with precision-engineered thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet the rigorous demands of your lab's high-temperature research.
Whether you are developing next-generation potassium-ion batteries or advanced porous materials, our furnaces provide the atmospheric purity and thermal uniformity necessary for success.
Ready to optimize your carbonization process? Contact our technical experts today to find the perfect furnace for your unique needs.
Visual Guide
References
- Can Li, Qingang Xiong. Bark‐Derived Oxygen‐Doped Porous Hard Carbon Anodes for Potassium‐Ion Batteries. DOI: 10.1002/ente.202402287
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- How does a high-temperature electric furnace facilitate the sintering process of 3Y-TZP ceramics? Master Densification
- What factors should be considered when purchasing an inert oven? Ensure Optimal Performance and Safety
- Why is a controlled atmosphere necessary in industrial debinding furnaces? Master the Switch from Nitrogen to Air
- Why is it necessary to use a high-purity argon gas protective atmosphere? Ensure Precision in Brazing Filler Melting
- What are some common gases and vapors used in furnace atmospheres? Optimize Your Heat Treatment Process
- How does an atmosphere furnace benefit the metallurgy industry? Enhance Material Quality and Efficiency
- What types of industries commonly use box-type atmosphere furnaces? Essential for Metallurgy, Electronics, and More
- What are the advantages of a hydrogen reducing atmosphere for stainless steel MIM parts? Achieve Superior Integrity



















