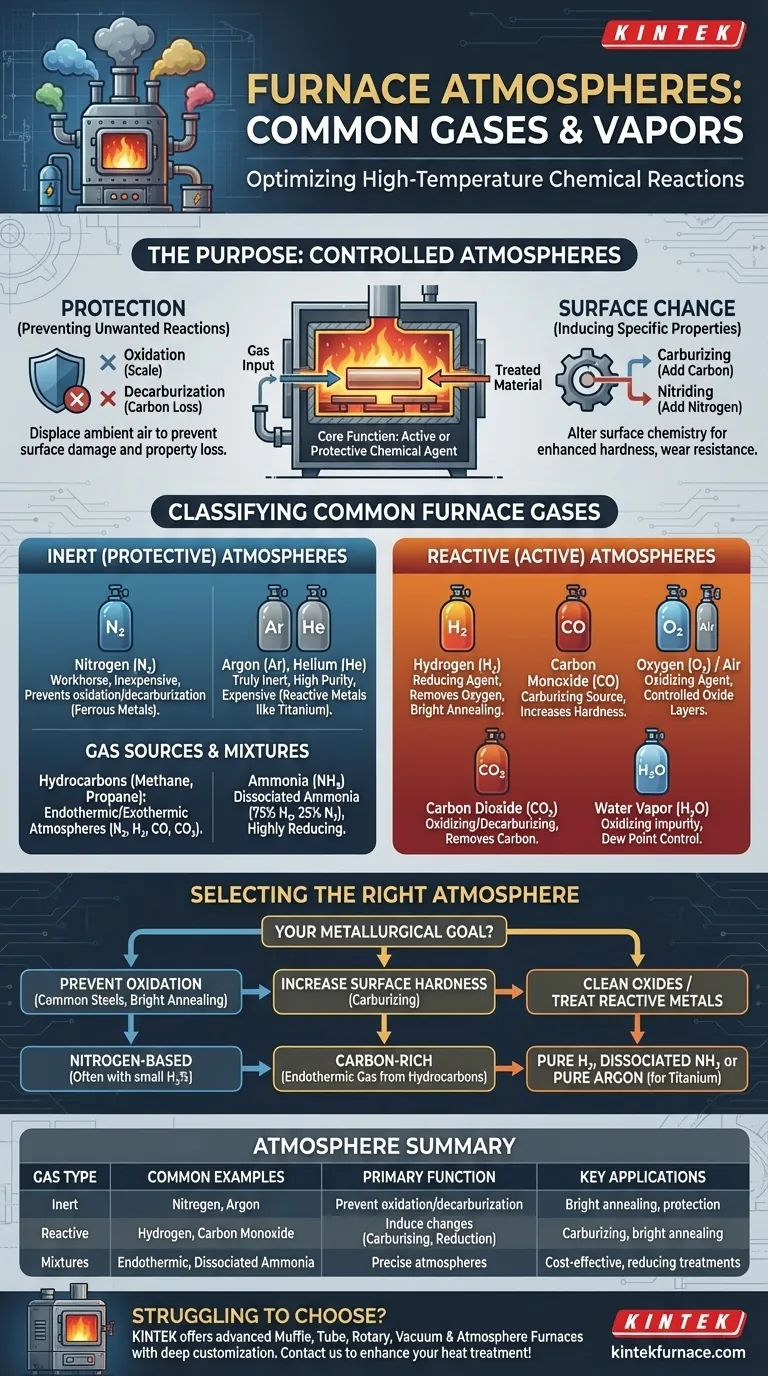
In industrial furnaces, the atmosphere is precisely controlled using a specific set of gases to manage chemical reactions on the material's surface at high temperatures. The most common gases are nitrogen, hydrogen, argon, carbon monoxide, and carbon dioxide, along with mixtures derived from ammonia or hydrocarbons like methane. The choice of gas is dictated by the desired outcome, whether it's to protect the material from chemical change or to intentionally alter its surface properties.
The core function of a furnace atmosphere is not merely to fill a space, but to serve as an active or protective chemical agent. Selecting the correct gas is critical for controlling the final properties of the workpiece, such as its hardness, corrosion resistance, and surface finish.

The Purpose of a Controlled Atmosphere
At the high temperatures found in heat-treating furnaces, most metals become highly reactive with the ambient air. Uncontrolled exposure to oxygen, moisture, and carbon dioxide can lead to undesirable outcomes.
Preventing Unwanted Reactions
The primary goal of many furnace atmospheres is protection. This involves displacing the normal ambient air to prevent two key problems:
- Oxidation: The formation of scale (metal oxides) on the surface, which can ruin the finish and dimensions of a part.
- Decarburization: The loss of carbon from the surface of steel, which reduces its hardness and wear resistance.
Inducing a Specific Surface Change
Conversely, some processes use a reactive atmosphere to intentionally alter the chemistry of the material's surface. This is done to enhance specific properties of the workpiece. Common examples include carburizing (adding carbon) or nitriding (adding nitrogen) to harden the surface of steel parts.
Classifying Common Furnace Gases
Furnace gases are best understood by their chemical behavior at high temperatures. They generally fall into two categories: inert (protective) or reactive (active).
Inert (Protective) Atmospheres
These gases are used to displace air and prevent chemical reactions.
- Nitrogen (N₂): The workhorse of protective atmospheres. It is relatively inexpensive and inert in most ferrous metal applications, effectively preventing oxidation and decarburization.
- Inert Gases (Argon, Helium): These are truly inert under all conditions. Argon is heavier than air and excellent for purging. While providing the purest protection, their high cost limits their use to applications involving highly reactive metals (like titanium) or when absolute purity is required.
Reactive (Active) Atmospheres
These gases are chosen specifically to react with the workpiece.
- Hydrogen (H₂): A powerful reducing agent. Its primary function is to react with and remove oxygen. It is highly effective at reducing surface oxides, resulting in a bright, clean surface finish, a process often called "bright annealing."
- Oxygen (O₂) and Air: An oxidizing agent. While often considered a contaminant, controlled amounts of oxygen or air are sometimes intentionally introduced to create a specific oxide layer on a material's surface.
- Carbon Monoxide (CO): A key component in carburizing atmospheres. It serves as a source of carbon, which diffuses into the surface of steel to increase its hardness.
- Carbon Dioxide (CO₂): Can be either carburizing or decarburizing depending on its equilibrium with Carbon Monoxide. In many contexts, it is considered an oxidizing agent that can remove carbon from steel.
- Water Vapor (H₂O): A common impurity that is highly reactive and typically acts as an oxidizing agent, especially at lower temperatures. The amount of water vapor is measured as the "dew point" and must be carefully controlled.
Gas Sources and Mixtures
Often, a specific mixture is generated rather than using pure gases.
- Hydrocarbons (Methane, Propane, Butane): These gases are not typically used directly but are reacted with air in a generator to produce "endothermic" or "exothermic" atmospheres—precise mixtures of N₂, H₂, CO, and CO₂.
- Ammonia (NH₃): Used as a source for nitrogen and hydrogen. When heated, "dissociated ammonia" breaks down into a mixture of 75% hydrogen and 25% nitrogen, creating a highly reducing atmosphere.
Understanding the Trade-offs and Risks
Choosing an atmosphere involves balancing effectiveness, cost, and safety. There is no single "best" gas, only the right gas for a specific application and budget.
Purity vs. Cost
A perfectly inert argon atmosphere provides superior protection but is expensive. For many steel-treating applications, a nitrogen-based atmosphere generated on-site is significantly more economical and provides sufficient protection.
Safety and Handling
Reactive gases introduce significant safety challenges. Hydrogen is highly flammable and explosive, while carbon monoxide is extremely toxic. Facilities using these gases require specialized handling equipment, robust safety protocols, and continuous monitoring.
Equipment and Atmosphere Control
The ability to maintain a pure atmosphere depends on the furnace design. A basic "purge and seal" furnace is economical but may struggle to achieve the very low dew points (low moisture) required for sensitive materials. A "retort" furnace, which isolates the workpiece in a sealed alloy container, offers superior atmosphere purity but at a higher initial and maintenance cost.
Selecting the Right Atmosphere for Your Process
Your choice of furnace atmosphere should be driven directly by your metallurgical goal.
- If your primary focus is preventing oxidation on common steels (bright annealing): A nitrogen-based atmosphere, often with a small percentage of hydrogen, offers the best balance of performance and cost.
- If your primary focus is increasing surface hardness (carburizing): You need a carbon-rich atmosphere, typically an endothermic gas generated from hydrocarbons to create a high potential of carbon monoxide.
- If your primary focus is cleaning surface oxides from sensitive materials: A pure, dry hydrogen or dissociated ammonia atmosphere is the most effective choice.
- If your primary focus is treating highly reactive metals (e.g., titanium): A pure inert gas like argon is non-negotiable to prevent catastrophic contamination.
Ultimately, the furnace atmosphere is a critical process variable that directly controls the chemistry, quality, and performance of your final product.
Summary Table:
| Gas Type | Common Examples | Primary Function | Key Applications |
|---|---|---|---|
| Inert (Protective) | Nitrogen, Argon | Prevent oxidation and decarburization | Bright annealing of steels, protection of reactive metals |
| Reactive (Active) | Hydrogen, Carbon Monoxide | Induce surface changes like carburizing or oxide reduction | Carburizing for hardness, bright annealing for clean finishes |
| Gas Mixtures | Endothermic/Exothermic from hydrocarbons, Dissociated ammonia | Provide precise atmospheres for specific reactions | Cost-effective treatments, reducing atmospheres |
Struggling to choose the right furnace atmosphere for your lab? KINTEK specializes in advanced high-temperature furnace solutions, including Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. With our exceptional R&D and in-house manufacturing, we offer deep customization to precisely meet your unique experimental needs—ensuring optimal material properties and process efficiency. Contact us today to discuss how we can enhance your heat treatment processes!
Visual Guide
Related Products
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality
- What is the significance of nitrogen in atmosphere furnaces? Unlock Enhanced Heat Treatment and Surface Hardening
- What industries commonly use inert atmosphere heat treating? Key Applications in Military, Automotive, and More
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance
- What are the two main types of atmosphere furnaces and their characteristics? Choose the Right Furnace for Your Lab



















