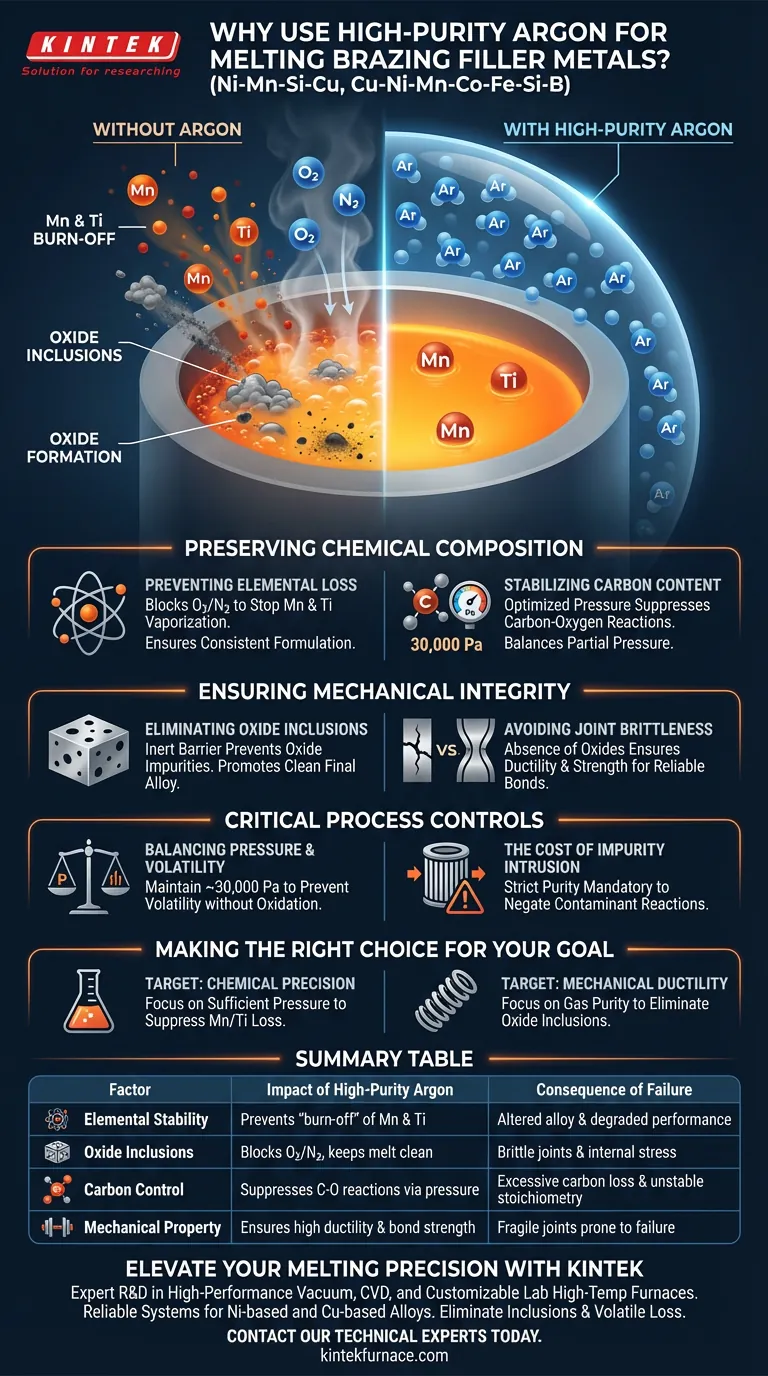
High-purity argon creates a critical inert barrier between the molten metal and the surrounding atmosphere. By isolating the melt from oxygen and nitrogen, this protective gas prevents the oxidation of reactive elements like manganese (Mn) and titanium (Ti). This ensures the final chemical composition remains consistent with design specifications and prevents the formation of oxide impurities that compromise the alloy's performance.
Using a high-purity argon atmosphere is not just about cleanliness; it is a fundamental requirement for maintaining the stoichiometry of the alloy. It prevents the loss of volatile active elements and suppresses carbon reactions, ensuring the filler metal retains the ductility and strength required for high-quality brazing.

Preserving Chemical Composition
Preventing Elemental Loss
Certain alloying elements, particularly manganese (Mn) and titanium (Ti), are highly reactive and volatile at melting temperatures.
Without a protective argon shield, these elements react rapidly with atmospheric oxygen. This results in the "burn-off" or loss of these critical components, altering the alloy's intended formulation and degrading its performance characteristics.
Stabilizing Carbon Content
In processes like vacuum induction melting, there is a risk of a carbon-oxygen reaction.
Maintaining an argon atmosphere at a specific pressure (approximately 30,000 Pa) helps suppress this reaction. This balances the partial pressure above the melt, preventing excessive carbon loss and ensuring the carbon content remains stable throughout the process.
Ensuring Mechanical Integrity
Eliminating Oxide Inclusions
When molten metal interacts with air, oxide impurities form instantly.
These oxides become trapped within the solidified filler metal as inclusions. A high-purity argon environment effectively blocks the intrusion of these impurities during the final stages of melting.
Avoiding Joint Brittleness
The presence of oxides and uncontrolled chemical shifts leads to inferior mechanical properties.
Specifically, oxide inclusions create stress points that significantly increase the brittleness of the brazed joint. By preventing oxidation, argon ensures the filler metal produces a ductile, robust bond rather than a fragile one.
Critical Process Controls
Balancing Pressure and Volatility
While the primary goal is exclusion of oxygen, the pressure of the argon atmosphere is also a critical variable.
If the pressure is too low, volatile elements may still evaporate even without oxidation. As noted in vacuum induction melting, maintaining a pressure around 30,000 Pa is necessary to suppress specific chemical reactions and stabilize the melt.
The Cost of Impurity Intrusion
Failing to maintain high purity in the argon gas itself can negate the benefits of the atmosphere.
Even trace amounts of contaminants in the gas supply can react with the melt at high temperatures. The system relies on the gas being strictly inert to guarantee that the final alloy matches the theoretical design.
Making the Right Choice for Your Goal
When establishing your melting parameters for Ni-Mn-Si-Cu or similar complex alloys, consider your specific quality targets:
- If your primary focus is Chemical Precision: Ensure the argon pressure is sufficient to suppress the vaporization and oxidation of volatile elements like Manganese.
- If your primary focus is Mechanical Ductility: Prioritize the purity of the argon gas to strictly eliminate oxide inclusions that cause brittleness.
Control the atmosphere, and you control the reliability of the final brazed joint.
Summary Table:
| Factor | Impact of High-Purity Argon | Consequence of Failure |
|---|---|---|
| Elemental Stability | Prevents "burn-off" of reactive Mn and Ti | Altered alloy formulation & degraded performance |
| Oxide Inclusions | Blocks oxygen/nitrogen to keep melt clean | Brittle joints and internal stress points |
| Carbon Control | Suppresses carbon-oxygen reactions via pressure | Excessive carbon loss and unstable stoichiometry |
| Mechanical Property | Ensures high ductility and bond strength | Fragile joints prone to failure |
Elevate Your Melting Precision with KINTEK
Don't let atmospheric contamination compromise your alloy performance. At KINTEK, we understand that maintaining chemical stoichiometry and mechanical integrity is non-negotiable for high-quality brazing filler metals.
Backed by expert R&D and manufacturing, we offer high-performance Vacuum, CVD, and customizable lab high-temp furnaces designed to maintain precise atmospheric controls and stable pressure environments. Whether you are melting complex Ni-based or Cu-based alloys, our systems provide the reliability you need to eliminate oxide inclusions and volatile element loss.
Ready to optimize your high-temperature processes? Contact our technical experts today to find the perfect customizable furnace solution for your unique laboratory needs.
Visual Guide
References
- S.V. Maksymova, P.V. Kovalchuk. Brazing stainless steel with high chromium nickel alloy. DOI: 10.21203/rs.3.rs-7259392/v1
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Magnesium Extraction and Purification Condensing Tube Furnace
People Also Ask
- What is the role of a benchtop drying oven in the preparation of Cu/TiO2/ZSM-5 catalysts? Ensure Optimal Dispersion
- Why is an air furnace used with boron nitride powder for copper? Achieve Oxidation-Free Heat Treatment
- What advantages does argon have over other inert gases in heat treatment? Superior Protection for Reactive Metals
- What are the vacuum capabilities of a controlled atmosphere furnace? Essential for Precise Gas Environment Control
- How do high-precision heating furnaces and nitrogen protection contribute to HTXRD? Optimize Your In-Situ Analysis
- What critical environmental controls do furnaces provide for ceramic 4D printing? Achieve Perfect Debinding Results
- What is an atmosphere furnace used for? Achieve Precise Material Processing in Controlled Environments
- How does a horizontal box furnace facilitate atmosphere control in the synthesis of Ni-TiON catalysts?















