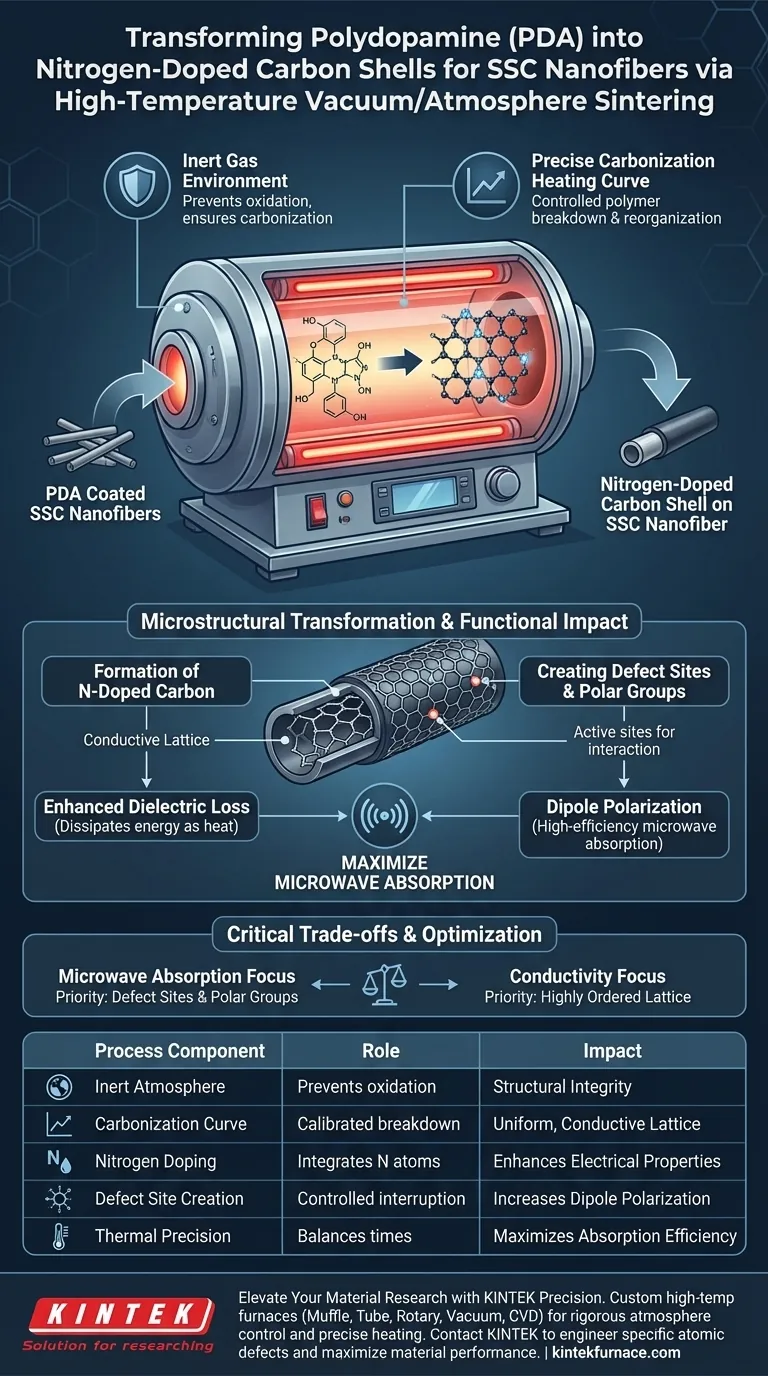
High-temperature atmosphere sintering furnaces convert Polydopamine (PDA) into nitrogen-doped carbon shells by subjecting the material to a strictly controlled inert gas environment and a specific carbonization heating curve. This thermal process reorganizes the organic PDA structure into a conductive carbon lattice while simultaneously generating defect sites that are essential for electromagnetic performance.
The furnace’s primary role is not merely heating, but precisely controlling the carbonization environment to create defect-rich, conductive shells. This transformation is critical for enhancing dielectric loss and dipole polarization, which ultimately maximizes the material's microwave absorption capabilities.

The Role of Controlled Atmosphere and Heating
To successfully transform PDA into a functional shell for Silicon Carbide (SSC) nanofibers, the furnace must maintain rigorous environmental conditions.
Inert Gas Environment
The sintering process takes place within an inert gas environment. This prevents the PDA from simply burning away (oxidizing) at high temperatures. Instead of combusting, the material undergoes carbonization, shedding non-carbon elements while retaining the necessary structural integrity.
The Precise Heating Curve
The transformation relies on a precise carbonization heating curve. The rate at which the temperature rises and holds is calibrated to control exactly how the polymer chains break down and reorganize. This precision ensures the formation of a uniform shell rather than a disordered or brittle coating.
Microstructural Transformation
The physical properties of the PDA layer change fundamentally during this process.
Formation of Nitrogen-Doped Carbon
As the PDA carbonizes, it transforms into a conductive nitrogen-doped carbon shell. Because PDA naturally contains nitrogen, the sintering process integrates these nitrogen atoms into the carbon lattice. This "doping" alters the electrical properties of the shell, making it conductive.
Creating Defect Sites and Polar Groups
The furnace control allows for the intentional creation of abundant defect sites and polar groups. In the context of material science, these are not flaws; they are active sites where the atomic structure is interrupted or unbalanced. These sites are crucial for the material's interaction with electromagnetic waves.
Functional Impact on Performance
The structural changes driven by the furnace directly translate to the material's ability to absorb microwaves.
Enhancing Dielectric Loss
The presence of the nitrogen-doped carbon shell significantly enhances the dielectric loss capacity of the material. This refers to the material's ability to dissipate electromagnetic energy as heat. The conductive nature of the carbon shell is the primary driver of this loss mechanism.
Dipole Polarization
The defect sites and polar groups created during sintering introduce dipole polarization. When exposed to microwaves, these polar groups attempt to align with the electromagnetic field. This molecular friction facilitates high-efficiency microwave absorption.
Understanding the Trade-offs
While high-temperature sintering is effective, it requires a delicate balance of parameters.
Sensitivity to Heating Rates
If the heating curve is not followed precisely, the carbonization may be incomplete or excessive. Inconsistent heating can lead to a lack of defect sites, reducing the material's absorption capabilities, or structural failure of the shell.
Balancing Conductivity and Defects
There is a trade-off between pure conductivity and the number of defect sites. A perfectly crystalline carbon structure is highly conductive but may lack the polar groups needed for dipole polarization. The furnace process must strike the right balance to maximize both dielectric loss and polarization.
Making the Right Choice for Your Goal
When configuring your sintering process for SSC nanofibers, consider your specific performance targets.
- If your primary focus is Microwave Absorption: Prioritize a heating curve that maximizes the creation of defect sites and polar groups to enhance dipole polarization.
- If your primary focus is Conductivity: Focus on ensuring a stable inert atmosphere to facilitate the formation of a continuous, highly ordered nitrogen-doped carbon lattice.
The success of converting PDA into a functional shell lies in using the furnace to engineer specific atomic defects rather than just achieving high temperatures.
Summary Table:
| Process Component | Role in PDA Conversion | Impact on SSC Performance |
|---|---|---|
| Inert Atmosphere | Prevents oxidation/combustion | Ensures structural integrity of the shell |
| Carbonization Curve | Calibrated polymer breakdown | Creates uniform, conductive carbon lattices |
| Nitrogen Doping | Integrates N atoms into lattice | Enhances conductivity and electrical properties |
| Defect Site Creation | Controlled structural interruption | Increases dipole polarization for microwave absorption |
| Thermal Precision | Balances heat and hold times | Maximizes dielectric loss and absorption efficiency |
Elevate Your Material Research with KINTEK Precision
Ready to achieve the perfect carbonization curve for your SSC nanofibers? KINTEK provides industry-leading Muffle, Tube, Rotary, Vacuum, and CVD systems designed for the most demanding thermal processes. Backed by expert R&D and manufacturing, our high-temperature furnaces offer the rigorous atmosphere control and precise heating profiles required to engineer specific atomic defects and nitrogen-doped structures.
Don't settle for inconsistent results—contact KINTEK today to customize a high-temp furnace solution tailored to your unique lab needs and maximize your material performance!
Visual Guide
References
- Limeng Song, Rui Zhang. Heterointerface‐Engineered SiC@SiO <sub>2</sub> @C Nanofibers for Simultaneous Microwave Absorption and Corrosion Resistance. DOI: 10.1002/advs.202509071
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What is the range of carburizing temperatures in vacuum carburizing? Optimize for Speed and Quality
- How does electrode design influence the thermal field stability? Optimize Heat Uniformity in Vacuum Sintering
- What role does an industrial-grade vacuum sintering furnace play in the final molding of 17-4 PH stainless steel parts?
- How does vacuum tempering contribute to energy efficiency? Superior furnace design slashes thermal waste and cuts costs.
- What processes are hot wall vacuum furnaces used for? Ideal for precise, uniform low-temperature heat treatments
- What safety measures are important for vacuum annealing furnaces? Ensure Reliable Operation and Protect Your Lab
- What role does a vacuum system play in Vanadium-Nitrogen alloy preparation? Boost Chemical Efficiency and Yield
- How does vacuum sintering improve surface finish? Achieve Superior, Oxide-Free Results



















