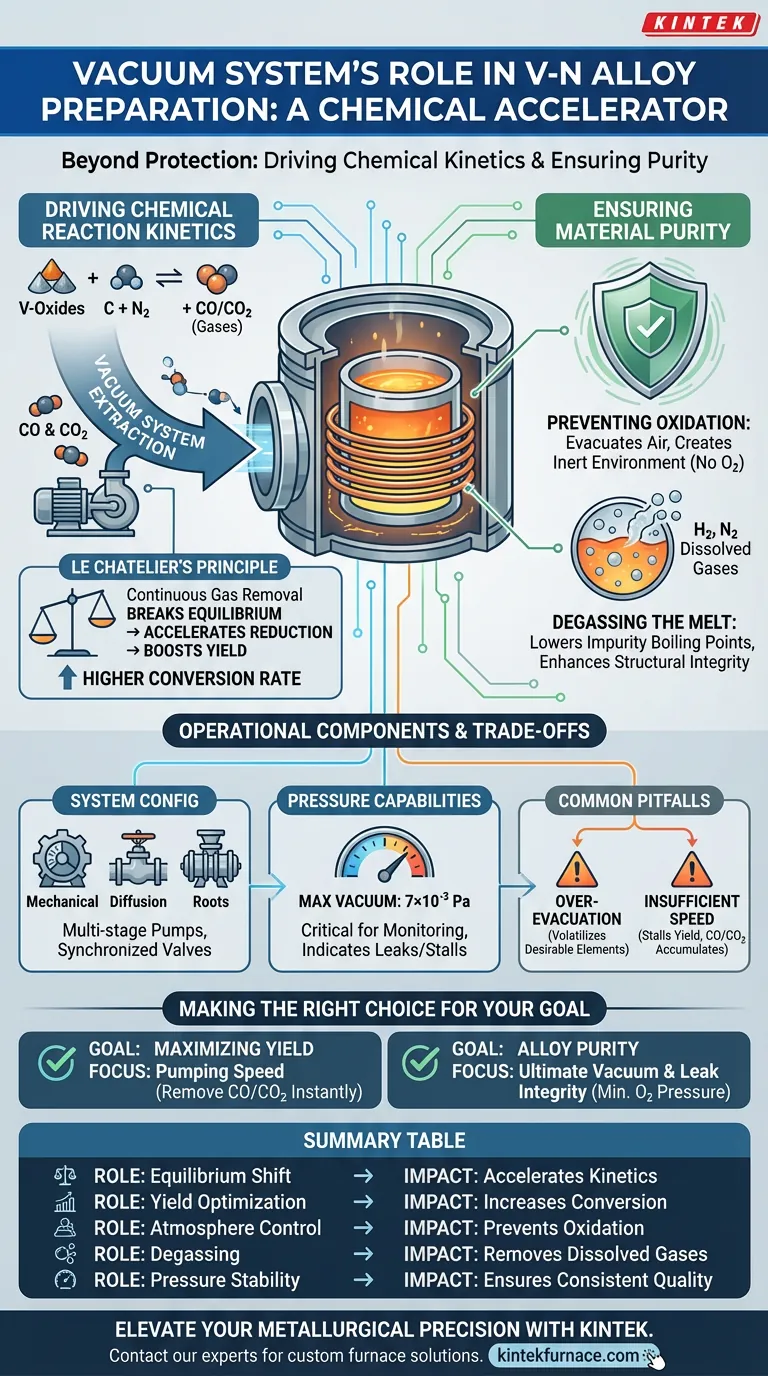
The role of a vacuum system in preparing Vanadium-Nitrogen alloys extends far beyond simply creating a clean environment; it is an active driver of chemical efficiency. Specifically, the system removes exhaust gases like carbon monoxide (CO) and carbon dioxide (CO2) generated during reaction nodes. By eliminating these byproducts, the system shifts the chemical equilibrium, forcing the reduction reaction to proceed rapidly toward the formation of the target nitride and significantly improving product yield.
Core Insight: The vacuum system is not just a protective shield; it is a chemical accelerator. By continuously evacuating gaseous reaction products, it leverages Le Chatelier's principle to break equilibrium, driving the transformation of raw materials into high-yield Vanadium-Nitrogen alloys.
Driving Chemical Reaction Kinetics
The primary function of the vacuum system in this specific application is to manipulate the thermodynamics of the furnace environment.
Shifting Chemical Equilibrium
In the reduction phase of Vanadium-Nitrogen alloy preparation, the reaction produces gases such as carbon monoxide and carbon dioxide.
If these gases are allowed to accumulate, the reaction reaches a state of equilibrium and stalls. The vacuum system continuously extracts these gases, lowering their partial pressure in the chamber.
Applying Le Chatelier's Principle
This removal process relies on Le Chatelier's principle. When the system removes the gaseous "products" of the reaction, the chemical balance is broken.
To restore balance, the system forces the reaction to consume more reactants. This drives the process forward, accelerating the conversion of high-valence vanadium oxides into low-valence oxides and eventually the desired alloy.
Improving Yield
The direct result of this chemical manipulation is a higher conversion rate.
By preventing the reaction from stagnating in a gaseous atmosphere of its own byproducts, the vacuum system ensures a more complete reduction. This leads to a higher overall yield of the final Vanadium-Nitrogen product.
Ensuring Material Purity
While reaction kinetics are the primary driver for this specific alloy, the vacuum system performs essential protective functions common to vacuum metallurgy.
Preventing Oxidation
Vanadium alloys have a high affinity for oxygen. Even trace amounts of atmospheric oxygen during the melting process can lead to contamination and structural weaknesses.
The vacuum system evacuates air to create an inert environment. This prevents the formation of unwanted oxides that would degrade the quality of the alloy.
Degassing the Melt
Beyond preventing surface oxidation, the vacuum aids in the removal of dissolved gases within the molten metal.
Gases like hydrogen and nitrogen (when not chemically bound) can be detrimental to the alloy's structural integrity. The vacuum environment lowers the boiling point of these impurities, allowing them to escape the melt effectively.
Operational Components and Trade-offs
Understanding the hardware limitations is crucial for optimizing the process.
System Configuration
A typical vacuum induction furnace utilizes a multi-stage pump configuration. This includes mechanical pumps, diffusion pumps, and Roots pumps.
These are synchronized via vacuum valves to achieve the specific pressure levels required at different stages of the heating and reaction cycle.
Pressure Capabilities
The effectiveness of the system depends on the ultimate vacuum level achievable. Generally, high-performance systems aim for a maximum vacuum level of 7×10⁻³ Pa.
Monitoring this pressure via precise vacuum measuring instruments is critical, as fluctuations can indicate a stalled reaction or a leak.
Common Pitfalls to Avoid
While a deep vacuum is generally beneficial, it introduces operational trade-offs.
Over-evacuation can potentially volatilize desirable volatile elements if the pressure drops too low at the wrong temperature. Conversely, insufficient pumping speed during the peak reaction phase will fail to remove CO/CO2 fast enough, stalling the yield regardless of the theoretical vacuum capacity.
Making the Right Choice for Your Goal
To maximize the effectiveness of your vacuum induction furnace, align your operational parameters with your specific outcome.
- If your primary focus is Maximizing Yield: Prioritize the pumping speed during the reduction phase to ensure CO and CO2 are removed the instant they are generated.
- If your primary focus is Alloy Purity: Focus on the ultimate vacuum level and leak integrity to ensure absolute minimal partial pressure of oxygen prior to heating.
The vacuum system is the engine that converts chemical potential into metallurgical reality.
Summary Table:
| Feature | Role in V-N Alloy Preparation | Impact on Process |
|---|---|---|
| Equilibrium Shift | Removes CO/CO2 exhaust gases | Accelerates reduction reaction kinetics |
| Yield Optimization | Prevents reaction stagnation | Increases final product conversion rate |
| Atmosphere Control | Eliminates atmospheric oxygen | Prevents unwanted oxidation and contamination |
| Degassing | Lowers impurity boiling points | Removes dissolved gases for structural integrity |
| Pressure Stability | Reaches up to 7×10⁻³ Pa | Ensures consistent metallurgical quality |
Elevate Your Metallurgical Precision with KINTEK
Don't let chemical equilibrium limit your production yield. KINTEK provides industry-leading vacuum induction furnace systems designed to accelerate reaction kinetics and ensure the highest material purity. Backed by expert R&D and manufacturing, we offer customizable Muffle, Tube, Rotary, Vacuum, and CVD systems tailored to your specific lab or industrial requirements.
Ready to optimize your Vanadium-Nitrogen alloy preparation? Contact our experts today to find your custom solution.
Visual Guide
References
- Xiaojie Cui, Yuekai Xue. Thermodynamic Study of Production of Vanadium–Nitrogen Alloy and Carbon Monoxide by Reduction and Nitriding of Vanadium Oxide. DOI: 10.3390/pr12091839
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- Why is gradient temperature control necessary in an infiltration furnace? Master Sintering Precision
- How are vacuum furnaces classified based on temperature? Find the Right Furnace for Your Heat Treatment Needs
- Why might a vacuum furnace maintain vacuum during cooling? Protect Workpieces from Oxidation and Control Metallurgy
- How is furnace brazing applied in the energy and power generation sectors? Enhance Component Reliability in Extreme Conditions
- What technical advantages do electric furnace systems offer for copper slag impoverishment? Maximize Your Metal Recovery
- Why are some vacuum furnaces backfilled with a partial pressure gas? Prevent Alloy Depletion in High-Temp Processes
- Why is a vacuum drying oven required for processing ball-milled red mud-alumina powder? Essential Drying Facts
- Why use a stainless steel autoclave with a PTFE liner for Ce-MOF synthesis? Ensure Safety and Purity



















