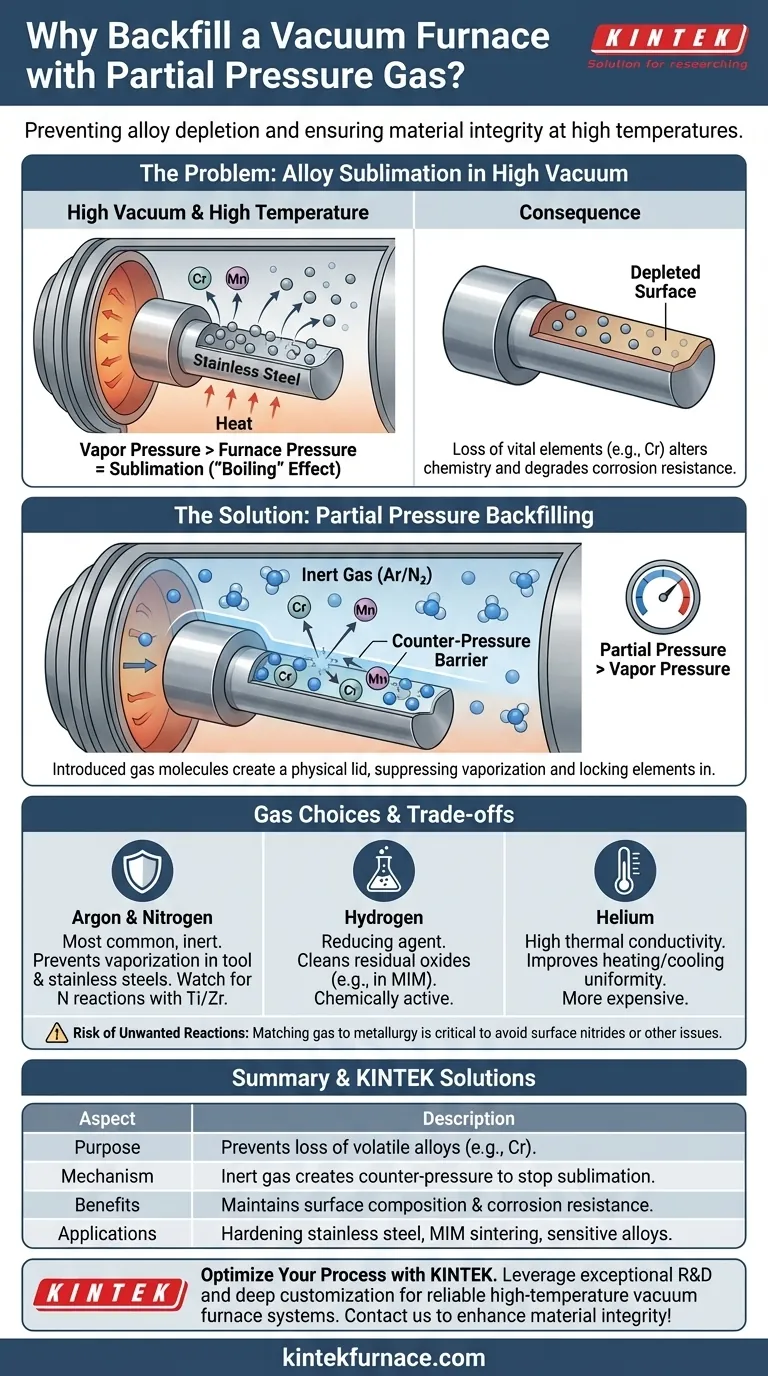
At its core, backfilling a vacuum furnace with a partial pressure gas is a precise technique used to prevent essential alloying elements from "boiling" off the surface of a part at high temperatures. This is especially critical during processes like hardening stainless steel, where the goal is to suppress the vaporization of chromium, which is vital for corrosion resistance.
The fundamental challenge is that a high vacuum makes it easy for certain metals to turn into a gas at high temperatures. Introducing a partial pressure of an inert gas acts as a physical barrier, creating just enough pressure to keep these volatile elements locked in the part's surface without compromising the benefits of the vacuum.

The Physics of Vapor Pressure in a Vacuum
To understand why backfilling is necessary, we must first look at the interplay between temperature, pressure, and the materials themselves.
The Nature of Vapor Pressure
Every solid and liquid has a vapor pressure, which is the natural pressure exerted by its gaseous form. This pressure increases exponentially as temperature rises.
At room temperature and normal atmospheric pressure, the vapor pressure of metals is negligible. However, in the high-heat, low-pressure environment of a vacuum furnace, this changes dramatically.
The Role of the Vacuum
A vacuum furnace works by removing air, which eliminates the risk of oxidation and removes other contaminants. Critically, it also removes atmospheric pressure.
This creates a massive pressure differential between the furnace environment and the vapor pressure of the alloying elements in the metal part.
The "Boiling" Effect at High Temperatures
When a part is heated in a vacuum, the vapor pressure of certain elements (like chromium, manganese, or copper) can exceed the extremely low pressure of the furnace.
This causes these elements to sublimate—transforming directly from a solid into a gas. This is functionally the same as water boiling when its vapor pressure exceeds atmospheric pressure. The elements literally boil off the part's surface.
The Consequence: Alloy Depletion
This isn't a minor effect. The loss of these elements from the surface can fundamentally alter the part's chemistry.
For a stainless steel part, the loss of surface chromium (chromium depletion) can severely degrade its corrosion resistance, defeating the purpose of using that alloy in the first place.
How Partial Pressure Backfilling Solves the Problem
Partial pressure backfilling is the elegant solution to this metallurgical challenge. It involves intentionally introducing a small, controlled amount of a specific gas back into the furnace chamber.
Creating a "Counter-Pressure"
The backfill gas, typically inert like Argon or Nitrogen, raises the total pressure inside the furnace.
This new pressure, while still far below atmospheric levels, is calculated to be just high enough to be greater than the vapor pressure of the volatile element you want to protect.
The Mechanism of Suppression
The molecules of the backfill gas act as a physical barrier. They bombard the surface of the part, effectively creating a "lid" that prevents the metal atoms from escaping into the vacuum.
This suppresses sublimation and keeps the alloy composition stable where it matters most: on the surface.
Understanding the Trade-offs and Gas Choices
The choice of backfill gas and pressure level is not arbitrary; it depends on the material being processed and the desired outcome.
Choosing the Right Gas
- Argon & Nitrogen: These are the most common choices for suppressing vaporization. They are inert, widely available, and highly effective for processes like hardening tool steels and stainless steels.
- Hydrogen: This gas is used when a chemical reaction is also desired. In processes like Metal Injection Molding (MIM), a hydrogen atmosphere not only provides partial pressure but also acts as a reducing agent, cleaning residual oxides from the parts.
- Helium: Due to its high thermal conductivity, helium can sometimes be used to improve the uniformity of heating and cooling, though it is a more expensive option.
The Risk of Unwanted Reactions
While "inert" gases are chosen to be non-reactive, at very high temperatures, even Nitrogen can react with certain metals. For example, nitrogen can form nitrides on the surface of titanium or zirconium alloys, which may be undesirable.
This highlights the importance of matching the gas and process parameters to the specific metallurgy of the component. The goal is a delicate balance—enough pressure to suppress vaporization, but not so much that it hinders outgassing or causes unwanted chemical reactions.
Making the Right Choice for Your Process
Applying partial pressure is a strategic decision based on your primary metallurgical goal.
- If your primary focus is preventing alloy depletion in steels: Use a partial pressure of Argon or Nitrogen calculated to exceed the vapor pressure of chromium at your target temperature.
- If your primary focus is sintering and oxide reduction (e.g., MIM): A partial pressure of Hydrogen is likely necessary to provide both a physical barrier and a chemically active reducing environment.
- If your primary focus is maximum purity for highly sensitive alloys: You may need to run at a harder vacuum and carefully limit your peak temperature to stay below the alloy’s critical vaporization point.
Ultimately, mastering partial pressure control allows you to leverage the full power of a vacuum while precisely safeguarding the material integrity of your components.
Summary Table:
| Aspect | Description |
|---|---|
| Purpose | Prevents vaporization of volatile alloy elements (e.g., chromium) at high temperatures in a vacuum environment. |
| Mechanism | Introduces inert gas (e.g., Argon, Nitrogen) to create a counter-pressure that suppresses sublimation. |
| Key Benefits | Maintains surface alloy composition, prevents corrosion resistance loss, and allows precise process control. |
| Common Gases Used | Argon, Nitrogen, Hydrogen (for reduction), Helium (for thermal uniformity). |
| Applications | Hardening stainless steel, sintering in MIM, processing sensitive alloys without depletion. |
Optimize your high-temperature processes with KINTEK's advanced vacuum furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with reliable high-temperature furnace systems, including Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures these solutions precisely meet your unique experimental needs, such as preventing alloy depletion with partial pressure control. Contact us today to discuss how we can enhance your material integrity and efficiency!
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- What is the necessity of using a vacuum chamber before curing epoxy? Eliminate Defects for Superior Material Integrity
- How does a vacuum furnace improve the mechanical properties of workpieces? Enhance Strength and Durability
- What are the main applications of vacuum furnaces? Achieve Superior Material Quality and Performance
- What is the purpose of an annealing furnace in the mechanical industry? Boost Metal Performance and Efficiency
- Why is a vacuum and atmosphere control system necessary for SiC sintering? Prevent Oxidation and Ensure High Purity
- What is the function of a heat treatment furnace in T4 treatment of SiC/Al? Enhance Composite Strength and Uniformity
- What are the technical advantages of using a vacuum environment for drying g-C3N4/Bi2WO6 catalysts?
- What are drop-bottom quench furnaces used for? Achieve Rapid Quenching for High-Performance Alloys



















