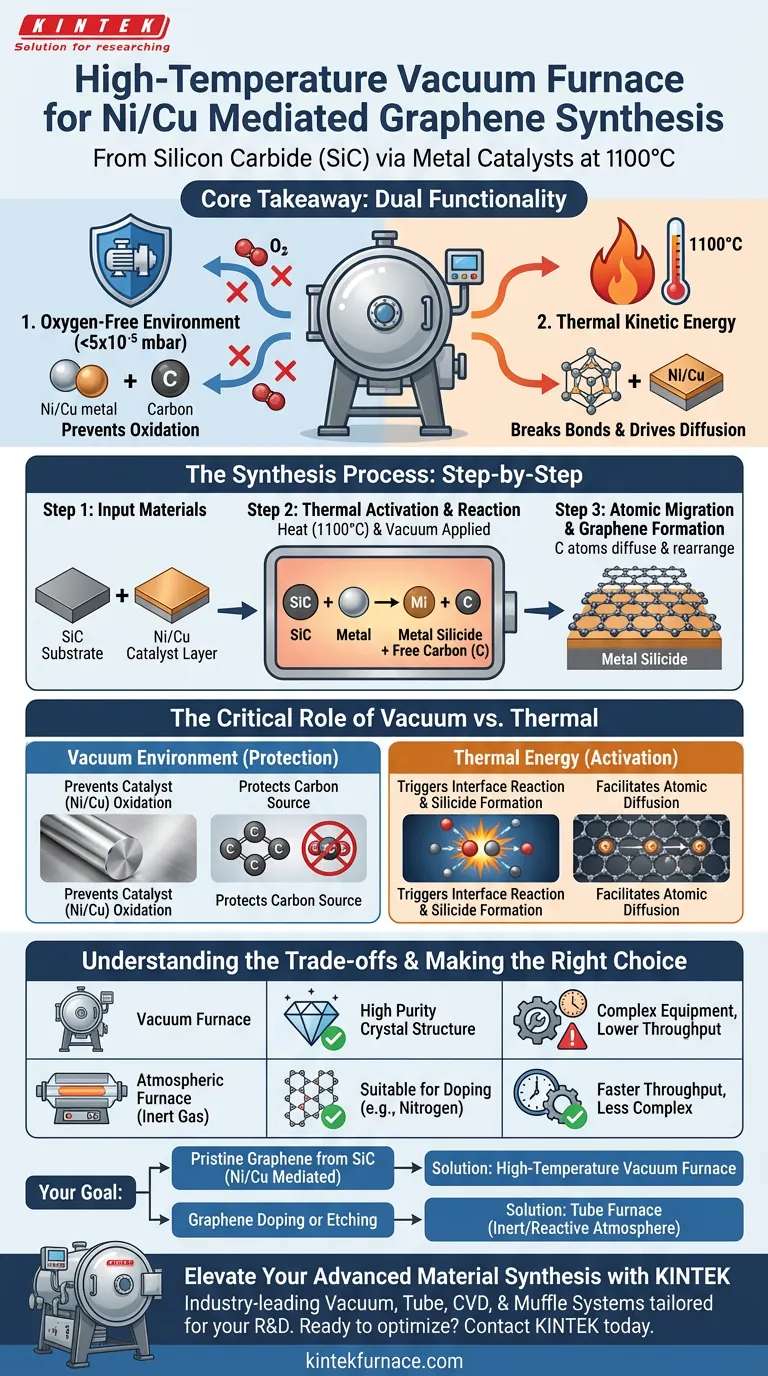
A high-temperature vacuum furnace facilitates the synthesis of graphene mediated by nickel and copper by creating the precise thermodynamic conditions required for an interfacial reaction between silicon carbide (SiC) and the metal layers. By heating the materials to 1100 °C within an ultra-high vacuum (typically below $5 \times 10^{-5}$ mbar), the furnace triggers the formation of metal silicides, which subsequently releases free carbon atoms to form the graphene structure.
Core Takeaway The vacuum furnace serves two simultaneous, critical functions: it provides the thermal kinetic energy necessary to break atomic bonds and drive diffusion, while strictly maintaining an oxygen-free environment to prevent the catastrophic oxidation of the metal catalysts and the carbon source.

The Critical Role of the Vacuum Environment
Prevention of Material Oxidation
The most immediate function of the furnace is the creation of a stable, ultra-high vacuum environment. When metals like nickel and copper are heated to synthesis temperatures (1100 °C), they become highly reactive to oxygen.
In an open atmosphere, these metals would undergo rapid, undesirable oxidation, ruining the catalyst surface. The vacuum chamber removes oxygen from the processing environment, ensuring the metals remain pure and active for the reaction.
Protecting the Carbon Source
Beyond protecting the metals, the vacuum is essential for the carbon itself. At these high temperatures, the liberated carbon atoms required to build the graphene lattice would instantly burn (oxidize) into carbon dioxide if oxygen were present. The vacuum ensures that the released carbon remains available for graphene formation.
Thermal Activation and Reaction Kinetics
Triggering the Interface Reaction
The furnace must maintain a temperature of approximately 1100 °C for a specified duration. This heat provides the necessary thermal kinetic energy to trigger the reaction at the interface of the silicon carbide and the metal layers.
Without this intense heat, the system would not possess the activation energy required to break the strong bonds within the silicon carbide.
Formation of Metal Silicides
Once the thermal threshold is crossed, the metal layers (nickel/copper) react with the silicon carbide. The furnace's sustained heat drives the formation of metal silicides.
This chemical transformation is the driver of the process: as the metal binds with the silicon, it forces the release of carbon atoms.
Facilitating Atomic Migration
High temperatures are also required for diffusion. As noted in diffusion annealing processes, maintaining constant high temperatures (850°C–1150°C) provides the activation energy for atoms to migrate across interfaces.
In this context, the thermal energy allows the released carbon atoms to rearrange themselves, transitioning from the bulk material to form the ordered, hexagonal lattice of graphene on the surface.
Understanding the Trade-offs
Equipment Complexity vs. Sample Purity
Using a high-vacuum furnace offers the highest purity environment, which is critical for determining precise material diffusion coefficients and achieving high-quality crystal structures.
However, this comes at the cost of complexity. Achieving and maintaining pressures below $5 \times 10^{-5}$ mbar requires sophisticated pumping systems and strict seal integrity compared to standard atmospheric tube furnaces.
Throughput Limitations
Vacuum processes are inherently batch-oriented and can be slower due to the time required to pump down the chamber and cool it safely.
While atmospheric furnaces (using inert gases like Argon) can be used for other graphene treatments—such as thermal reduction of graphene oxide or nitrogen doping—the specific SiC-to-graphene conversion mediated by metals relies on the strict oxidation control that only a high-vacuum furnace guarantees at 1100 °C.
Making the Right Choice for Your Goal
The selection of furnace technology depends heavily on the specific synthesis pathway and the quality of graphene required.
- If your primary focus is synthesizing pristine graphene from SiC via metal mediation: You must use a high-temperature vacuum furnace to prevent oxidation while enabling the metal silicide reaction at 1100 °C.
- If your primary focus is doping graphene (e.g., with nitrogen): A high-temperature tube furnace with an inert gas flow (Argon) is likely more suitable to facilitate the substitution of carbon atoms with dopants.
- If your primary focus is creating nanopores or chemical activation: A tube furnace capable of handling reactive atmospheres like CO2 or H2 is required to etch the material at controlled rates.
Success in graphene synthesis is defined not just by reaching the right temperature, but by precisely controlling the chemical atmosphere in which that heat is applied.
Summary Table:
| Feature | Vacuum Furnace Role in Graphene Synthesis | Key Outcome |
|---|---|---|
| Thermal Energy | Reaches 1100 °C to trigger SiC-metal interface reactions | Formation of metal silicides |
| Vacuum Level | Maintains < 5 x 10⁻⁵ mbar pressure | Prevents oxidation of catalyst and carbon |
| Diffusion Control | Sustains high activation energy for atomic migration | Ordered hexagonal graphene lattice |
| Environment | Oxygen-free processing chamber | High-purity crystal structure formation |
Elevate Your Advanced Material Synthesis with KINTEK
Achieving the precise thermodynamic conditions for graphene synthesis requires uncompromising thermal control and vacuum integrity. KINTEK provides industry-leading laboratory high-temperature furnaces—including Vacuum, Tube, CVD, and Muffle systems—engineered to meet the rigorous demands of your R&D.
Whether you are synthesizing pristine graphene, performing nitrogen doping, or developing custom chemical activation processes, our expert-backed manufacturing ensures your equipment is perfectly tailored to your unique research needs.
Ready to optimize your high-temperature reactions? Contact KINTEK today to discuss your custom furnace solution." Form)."
Visual Guide
References
- Aiswarya Pradeepkumar, Francesca Iacopi. Epitaxial graphene growth on cubic silicon carbide on silicon with high temperature neutron reflectometry: an <i>operando</i> study. DOI: 10.1039/d3ra08289j
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What are the key features of vacuum furnaces? Achieve Absolute Control for High-Performance Materials
- What are the common types of materials used for constructing heating elements in electrically heated vacuum furnaces? Choose the Right Material for Your Process
- What are the key specifications of vacuum carburizing furnaces? Optimize Your Heat Treatment Process
- Why is a high-temperature vacuum drying oven necessary for hard carbon? Protect Your Material Integrity
- What factors should be considered when choosing a vacuum furnace model? Key Insights for Optimal Performance
- What are the typical steps in vacuum sintering? Master High-Purity, Dense Material Production
- In which industries is vacuum brazing aluminum commonly applied? Essential for Aerospace, Automotive, Electronics, and Medical
- What are the components of a microwave sintering furnace? Boost Efficiency with Advanced Heating Systems



















