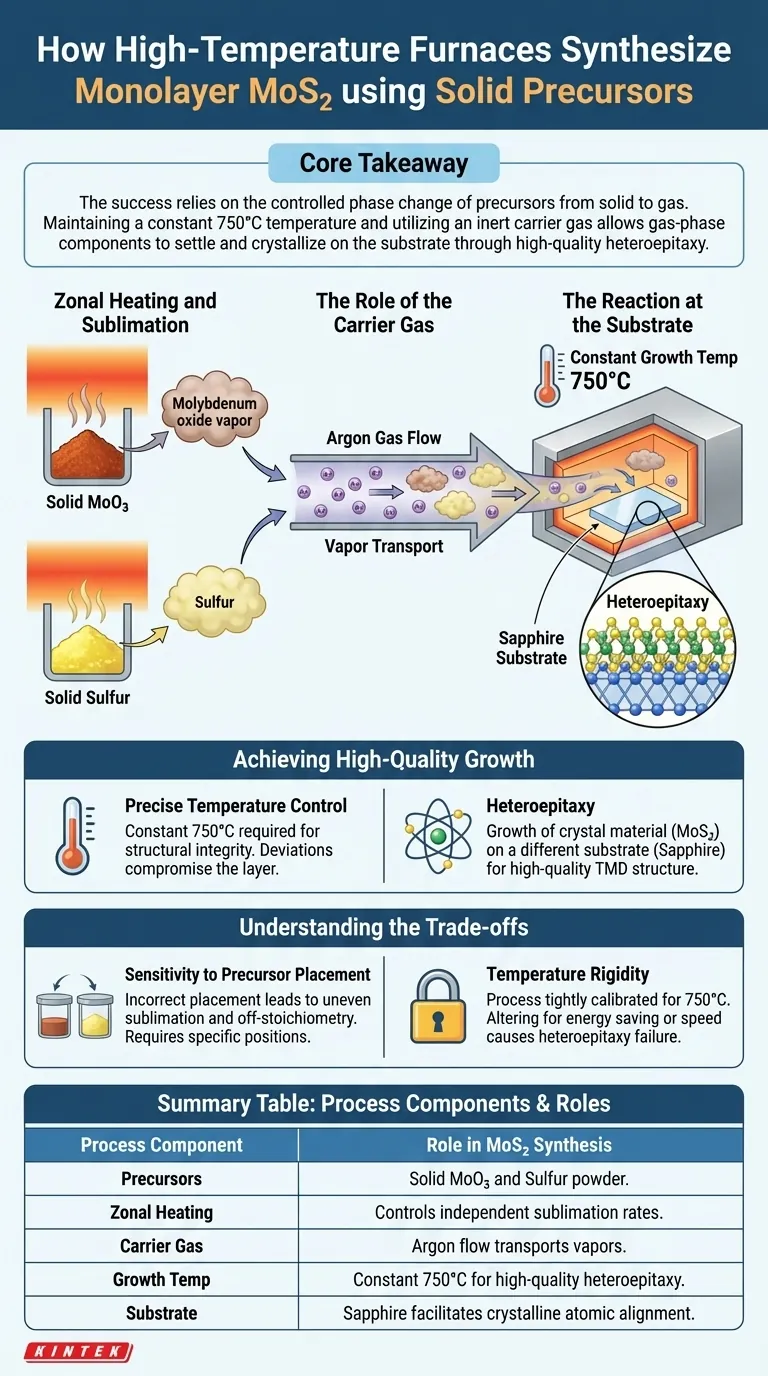
High-temperature furnaces synthesize monolayer molybdenum disulfide (MoS2) by precisely controlling the sublimation of solid powders. Specifically, the furnace uses zonal heating to vaporize solid precursors—powdered molybdenum trioxide (MoO3) and sulfur—placed at distinct locations. An argon gas flow then transports these vapors to a sapphire substrate, where they react at 750°C to form the atomic layer.
Core Takeaway The success of this synthesis relies on the controlled phase change of precursors from solid to gas. By maintaining a constant 750°C temperature and utilizing an inert carrier gas, the furnace enables the gas-phase components to settle and crystallize on the substrate through high-quality heteroepitaxy.

The Mechanics of Solid-Source Synthesis
Zonal Heating and Sublimation
The process begins with solid precursors, specifically molybdenum trioxide (MoO3) and sulfur powder.
Instead of heating the entire chamber uniformly, the furnace employs zonal heating. This allows the solid powders, placed at specific positions, to sublime (turn directly from solid to gas) at the appropriate rates required for the reaction.
The Role of the Carrier Gas
Once the solids have sublimated, they must be moved to the reaction site.
An argon gas flow acts as the transport vehicle. This inert gas guides the vaporized components through the reaction chamber, ensuring they reach the substrate rather than drifting aimlessly or depositing prematurely.
The Reaction at the Substrate
The synthesis target is a sapphire substrate located within the furnace.
When the gas-phase components reach this substrate, they undergo a chemical reaction. This results in the deposition of the monolayer MoS2 directly onto the sapphire surface.
Achieving High-Quality Growth
Precise Temperature Control
The furnace is critical for maintaining a specific thermal environment.
To ensure the formation of high-quality material, the furnace maintains a constant growth temperature of 750°C. Deviations from this temperature can compromise the structural integrity of the resulting layer.
Heteroepitaxy
The interaction between the reacting gases and the substrate is known as heteroepitaxy.
This process involves growing a crystal material (MoS2) on a different crystalline substrate (sapphire). The high temperature facilitates the alignment of the MoS2 atoms with the sapphire lattice, ensuring a high-quality transition metal dichalcogenide (TMD) structure.
Understanding the Trade-offs
Sensitivity to Precursor Placement
The reference highlights that precursors are placed at "specific positions."
Incorrect placement relative to the heating zones can lead to uneven sublimation. If the powders vaporize too quickly or too slowly, the stoichiometry of the gas mixture will be off, resulting in poor growth.
Temperature Rigidity
The process relies on a constant 750°C.
While this ensures quality for this specific reaction, it limits flexibility. The system is tightly calibrated for this temperature window, meaning significantly altering the temperature to save energy or speed up the process would likely result in failure of the heteroepitaxy.
Making the Right Choice for Your Goal
To replicate this synthesis successfully, you must prioritize process stability over speed.
- If your primary focus is Crystal Quality: strict adherence to the 750°C constant temperature is required to ensure proper heteroepitaxy on the sapphire.
- If your primary focus is Reaction Efficiency: Ensure the solid precursors are placed exactly where the zonal heating matches their sublimation points to maintain a steady supply of vapor.
Success depends on synchronizing the sublimation of solids with the precise thermal environment of the substrate.
Summary Table:
| Process Component | Role in MoS2 Synthesis |
|---|---|
| Precursors | Solid Molybdenum Trioxide (MoO3) and Sulfur powder |
| Zonal Heating | Controls independent sublimation rates of solid sources |
| Carrier Gas | Argon (Ar) flow transports vapors to the substrate |
| Growth Temp | Constant 750°C for high-quality heteroepitaxy |
| Substrate | Sapphire (facilitates crystalline atomic alignment) |
Elevate Your Material Synthesis with KINTEK
Precise MoS2 synthesis requires exact thermal zones and unwavering stability. KINTEK provides industry-leading CVD systems, Tube furnaces, and Vacuum solutions designed to handle the complexities of TMD growth. Backed by expert R&D and high-end manufacturing, our lab furnaces are fully customizable to meet your unique research requirements.
Ready to achieve superior crystal quality?
Contact our experts today to find the perfect high-temperature system for your laboratory.
Visual Guide
References
- Arash Vaghef‐Koodehi. Ultrasensitive Graphene-TMD Heterostructure Optical Biosensors Integrated with Silicon Photonics for Label-Free Detection. DOI: 10.21203/rs.3.rs-7279468/v1
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the function of a dual-zone tube furnace in LPCVD? Master Precise MnSe Nanosheet Synthesis
- What are the advantages of different heating zone configurations in tube furnaces? Optimize Your Thermal Processes
- How does high-temperature tube furnace programmed control influence porous carbon? Expert Pore Geometry Insights
- What role does a high-temperature tube furnace play in graphite recycling? Restoring Purity and Structure
- What types of atmospheres can a horizontal electric furnace control? Master Material Processing with Precision
- What role does a vacuum tube furnace play in the 600°C high-temperature annealing of Pd/TaTiNbZr/Ta multilayer membranes?
- How does the design of an electrically heated cylindrical reaction chamber influence nitriding for AISI 1085 steel?
- What are the key application features of a fluidized bed vertical tube furnace? Boost Efficiency and Uniformity



















