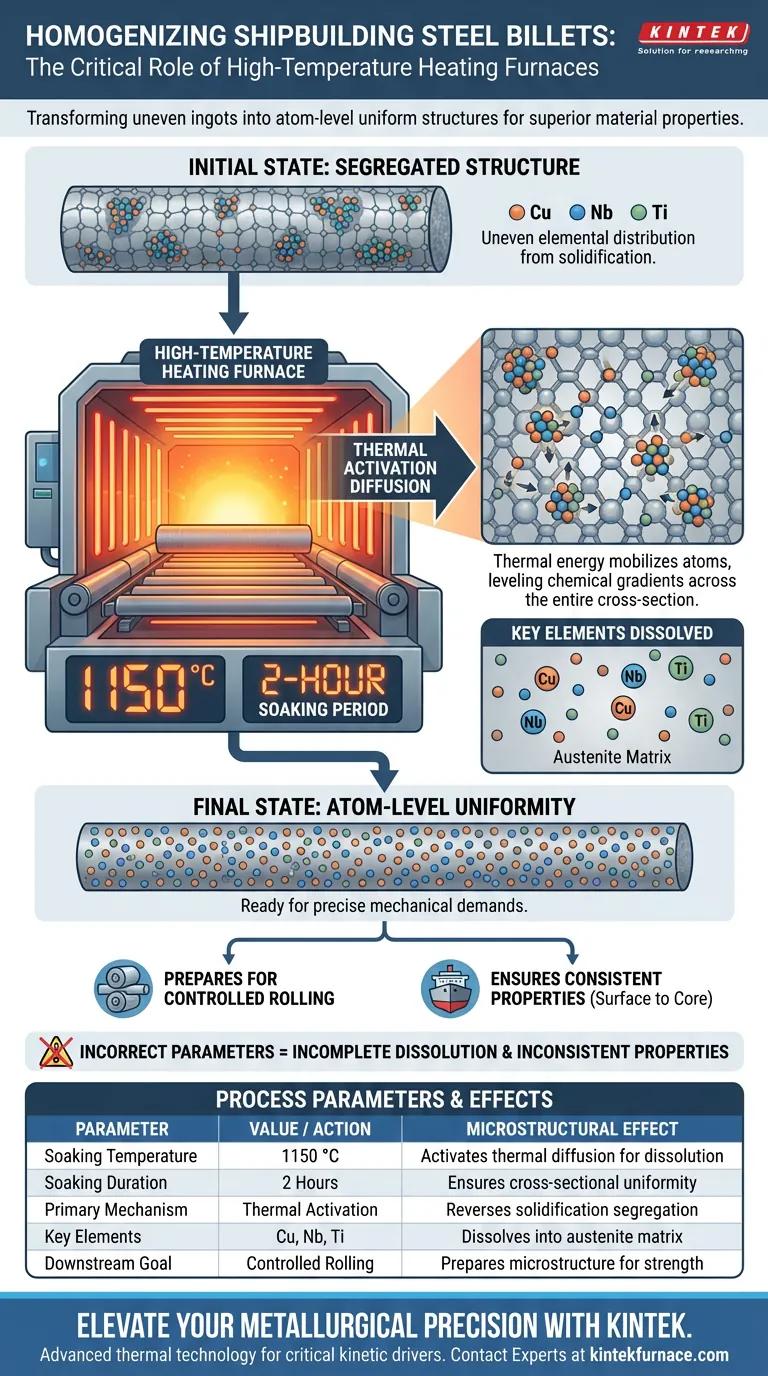
High-temperature heating acts as a critical kinetic driver for material uniformity in shipbuilding steel. By heating steel ingots to 1150 °C and maintaining this temperature for a 2-hour soaking period, the furnace utilizes high-temperature thermal activation diffusion to dissolve segregated micro-alloying elements into the austenite matrix.
The core purpose of this process is to convert the uneven elemental distribution of raw ingots into an atom-level uniform structure. By fully dissolving key elements like copper and niobium, the furnace prepares the steel's microstructure for the precise mechanical demands of subsequent controlled rolling.
The Mechanism of Homogenization
Thermal Activation Diffusion
The primary mechanism at work is high-temperature thermal activation diffusion.
At 1150 °C, the thermal energy provided to the steel lattice is sufficient to mobilize atoms.
This energy allows atoms to migrate from areas of high concentration to areas of low concentration, effectively leveling out chemical gradients.
The Role of the Soaking Period
Achieving the target temperature is only the first step; maintaining it is equally vital.
The 2-hour soaking period ensures that the thermal activation penetrates the entire cross-section of the billet.
This duration allows sufficient time for the diffusion process to complete, ensuring the center of the ingot is as homogenized as the surface.
Redistribution of Alloying Elements
Reversing Solidification Segregation
When steel ingots first solidify, elements naturally segregate, creating clusters of uneven composition.
The heating furnace reverses this natural segregation.
It redistributes these elements from their clumped state into an atom-level uniform distribution.
Dissolving Critical Micro-Alloys
Shipbuilding steel relies on specific micro-alloying elements for its strength and durability.
The process specifically targets copper, niobium, and titanium.
The furnace ensures these elements are fully dissolved into the austenite matrix, which is a prerequisite for their effectiveness in later processing stages.
Understanding Process Dependencies
The Link to Controlled Rolling
This heating phase cannot be viewed in isolation; it is a preparatory step.
The homogenization is specifically designed to prepare the microstructure for subsequent controlled rolling.
If the elements are not fully dissolved here, they cannot precipitate correctly during the rolling phase to strengthen the steel.
Adherence to Parameters
Success depends strictly on adhering to the specific time and temperature parameters.
Falling short of 1150 °C may result in incomplete dissolution of the niobium or titanium.
Similarly, cutting the 2-hour soak short risks leaving the core of the billet segregated, leading to inconsistent material properties.
Optimizing for Microstructural Integrity
To ensure the steel billet meets the rigorous standards required for shipbuilding, you must strictly control the thermal inputs.
- If your primary focus is complete element dissolution: Ensure the furnace temperature reaches and maintains a minimum of 1150 °C to activate diffusion in copper, niobium, and titanium.
- If your primary focus is cross-sectional uniformity: Strictly enforce the 2-hour soaking period to allow diffusion mechanisms to equalize the chemistry from surface to core.
Precise thermal management during this stage creates the fundamental chemical homogeneity required for high-performance steel.
Summary Table:
| Process Parameter | Target Value / Action | Microstructural Effect |
|---|---|---|
| Soaking Temperature | 1150 °C | Activates thermal diffusion for micro-alloy dissolution |
| Soaking Duration | 2 Hours | Ensures cross-sectional uniformity from surface to core |
| Primary Mechanism | Thermal Activation | Reverses solidification segregation at an atomic level |
| Key Elements | Cu, Nb, Ti | Dissolves alloying elements into the austenite matrix |
| Downstream Goal | Controlled Rolling | Prepares microstructure for precise mechanical properties |
Elevate Your Metallurgical Precision with KINTEK
Uniformity is the foundation of high-performance shipbuilding steel. KINTEK provides the advanced thermal technology required to master these critical kinetic drivers. Backed by expert R&D and manufacturing, we offer a wide range of Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as other lab high-temperature furnaces—all fully customizable to your specific heating parameters and soaking requirements.
Don't let inconsistent thermal distribution compromise your material integrity. Partner with KINTEK for reliable, high-precision heating solutions tailored to your unique research and production needs.
Contact Our Experts Today to Optimize Your Thermal Processes
Visual Guide
References
- Dian Zhang, Zhongran Shi. Effect of Reheating Temperature on the Microstructure and Properties of Cu-Containing 440 MPa Grade Non-Tempered Ship Plate Steel. DOI: 10.3390/ma17071630
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- What role does a muffle furnace play in the preparation of expanded graphite? Achieve Maximum Expansion through Thermal Shock
- Why is temperature control important in a muffle furnace? Ensure Accurate, Repeatable Results
- What role does a muffle furnace play in the synthesis of Nd:SrLaGaO4 crystal precursors? Precision Thermal Stability
- What is the significance of high-temperature calcination in a muffle furnace? Mastering Ce-TiO2 Catalyst Preparation
- What role does a high-temperature muffle furnace play in the preparation of a BiVO4 seed layer? Expert Synthesis Guide
- What is the significance of muffle furnaces in the ceramics industry? Unlock Precision and Purity for Superior Ceramics
- What are the operational advantages of box type high-temperature resistance furnaces? Achieve Reliable, User-Friendly Thermal Processing
- What role does a high-temperature box furnace play in the pre-calcination of LLZTO? Master Garnet Phase Synthesis



















