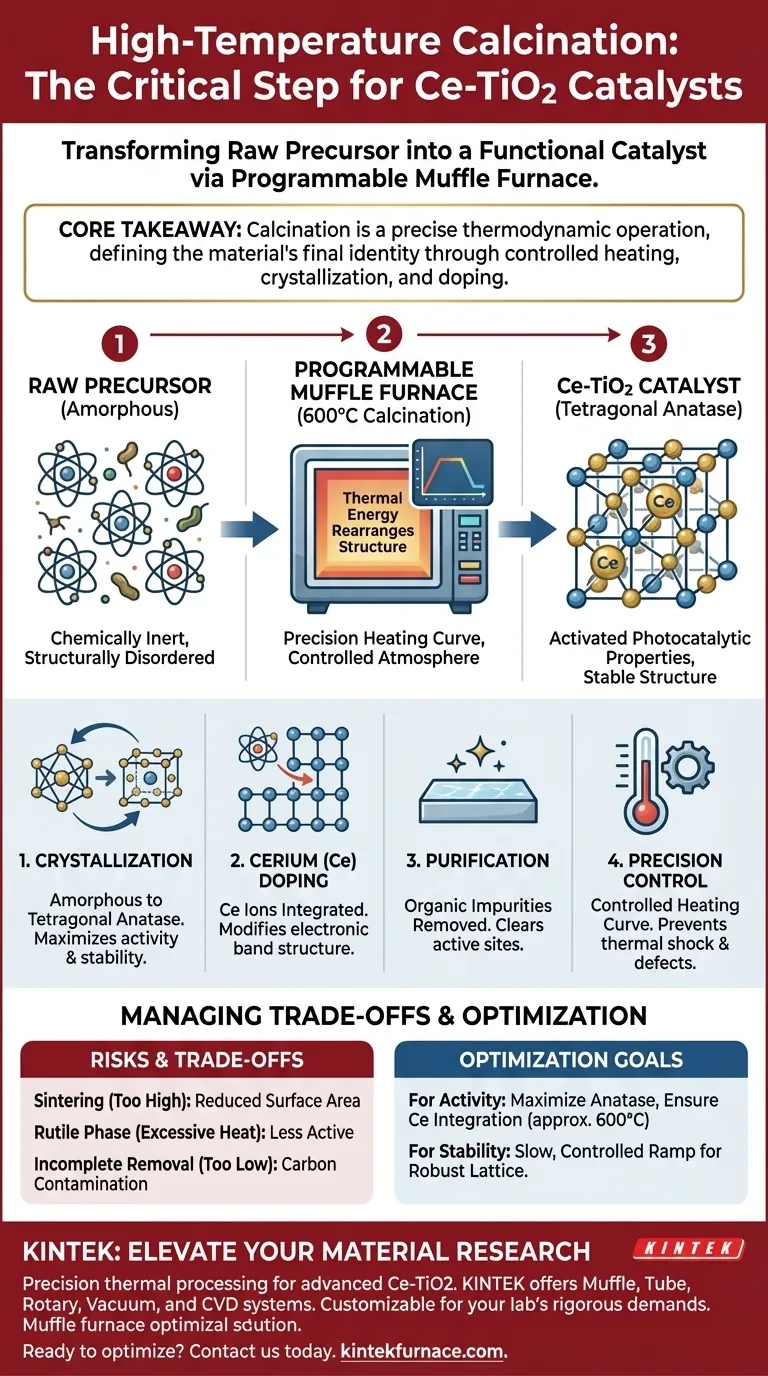
High-temperature calcination represents the critical phase-transition step where your raw chemical mixture becomes a functional catalyst. In the preparation of cerium-doped titanium dioxide (Ce-TiO2), this process—typically executed at 600°C in a programmable muffle furnace—transforms the amorphous precursor into a stable tetragonal anatase structure, removes organic impurities, and locks the cerium ions into the crystal lattice to activate photocatalytic properties.
Core Takeaway The calcination process is not merely about drying; it is a precise thermodynamic operation that defines the material's final identity. By strictly controlling the heating curve, you facilitate the crystallization of the anatase phase and the effective incorporation of cerium ions, which are the two primary drivers of the catalyst's physicochemical stability and performance.

Transforming Material Structure
The primary function of the muffle furnace in this context is to drive a specific crystallographic transformation. Without this thermal treatment, the material remains chemically inert and structurally disordered.
From Amorphous to Crystalline
Initially, the Ce-TiO2 precursor exists as an amorphous (shapeless) solid. The high thermal energy provided by the furnace rearranges the atomic structure into an ordered state.
Specifically, the heat drives the formation of the tetragonal anatase structure. This specific crystal phase is crucial because anatase generally exhibits higher photocatalytic activity than other titanium dioxide phases (such as rutile) for many applications.
The Role of Precision Heating
The "programmable" aspect of the furnace is vital. A precise heating curve ensures that energy is supplied at a controlled rate.
This control prevents thermal shock and allows the atoms sufficient time to diffuse and arrange themselves correctly. It ensures the crystal lattice forms with minimal defects, leading to a more robust final material.
Activation via Doping and Purification
Beyond simple crystallization, the high-temperature environment dictates the chemical purity and the electronic behavior of the catalyst.
Embedding Cerium Ions
The presence of Cerium (Ce) is what differentiates this catalyst from standard TiO2. Calcination facilitates the diffusion of Ce ions into the material.
The heat energy allows Ce ions to either embed directly into the titanium dioxide lattice or form active sites at the grain boundaries. This integration is what modifies the electronic band structure, enhancing the material's ability to facilitate photocatalytic reactions.
Elimination of Impurities
The precursor material often contains residual organic compounds, such as solvents or ligands used during the initial mixing stage.
The oxidation environment within the muffle furnace ensures these organic impurities are completely decomposed and removed. Eliminating these residues is non-negotiable, as they would otherwise block active sites and degrade the catalyst's performance.
Understanding the Trade-offs
While calcination is essential, the parameters must be balanced carefully to avoid degrading the catalyst.
The Risk of Sintering
If the temperature is too high or held for too long, the individual particles may sinter (fuse together). Sintering drastically reduces the specific surface area of the catalyst, leaving fewer active sites available for reactions.
Phase Transition Dangers
While 600°C promotes the anatase phase, excessive heat can drive the material toward the rutile phase. While stable, rutile is often less active for certain photocatalytic applications than anatase. Precision in the maximum temperature is critical to maintain the desired phase composition.
Incomplete Removal
Conversely, if the temperature is too low or the duration too short, organic residues may remain. This leads to carbon contamination on the surface, which interferes with light absorption and reactant adsorption.
Making the Right Choice for Your Goal
The specific parameters of your calcination program should be tuned based on your final performance metrics.
- If your primary focus is Photocatalytic Activity: Prioritize a temperature (around 600°C) that maximizes the anatase phase while ensuring enough heat is applied to fully integrate the cerium ions into the lattice.
- If your primary focus is Structural Stability: Ensure the heating ramp is slow and controlled to minimize structural defects, creating a mechanically robust crystal lattice that can withstand long-term use.
By mastering the thermal history of your precursor in the muffle furnace, you turn a simple mixture of powders into a highly efficient, engineered surface ready for complex catalysis.
Summary Table:
| Process Objective | Key Transformation | Why It Matters |
|---|---|---|
| Crystallization | Amorphous to Tetragonal Anatase | Maximizes photocatalytic activity and material stability. |
| Doping Activation | Cerium (Ce) Ion Integration | Modifies electronic band structure for enhanced catalysis. |
| Purification | Removal of Organic Impurities | Clears active sites and prevents catalyst degradation. |
| Precision Control | Controlled Heating Curve | Prevents thermal shock and minimizes crystal lattice defects. |
Elevate Your Material Research with KINTEK
Precision in thermal processing is the difference between a failed precursor and a high-performance catalyst. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of your laboratory.
Our programmable high-temperature furnaces provide the exact heating curves and temperature uniformity required for:
- Advanced Ce-TiO2 catalyst preparation
- Critical phase-transition control
- Customized lab-scale manufacturing
Ready to optimize your calcination process? Contact us today to explore our customizable furnace solutions and find the perfect fit for your unique research needs.
Visual Guide
References
- H.R. Khan. Cerium-Doped Titanium Dioxide (CeT) Hybrid Material, Characterization and Spiramycin Antibiotic Photocatalytic Activity. DOI: 10.3390/catal15060512
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the function of a Muffle Furnace in the production of cristobalite alpha silica nanoparticles? Precision 900°C Annealing
- What design features contribute to the durability of a muffle furnace? Discover Key Engineering for Long-Lasting Performance
- What is the role of a muffle furnace in the synthesis of Gold Nanoparticles supported on Bamboo Biochar (Au-NPs/BC)?
- Why is precise temperature control important in a muffle furnace? Ensure Reliable Results in Heat Treatment
- What role does a box muffle furnace play in life sciences? Unlock Precise Mineral Analysis in Research
- How do box resistance furnaces facilitate the optimization of mechanical properties in AlSi10Mg alloys? Expert Thermal Analysis
- What is the function of a high-temperature box-type resistance furnace in rGO synthesis? Optimize Your Carbonization
- What are the benefits of programmable controls in a muffle furnace? Unlock Precision and Automation for Your Lab



















