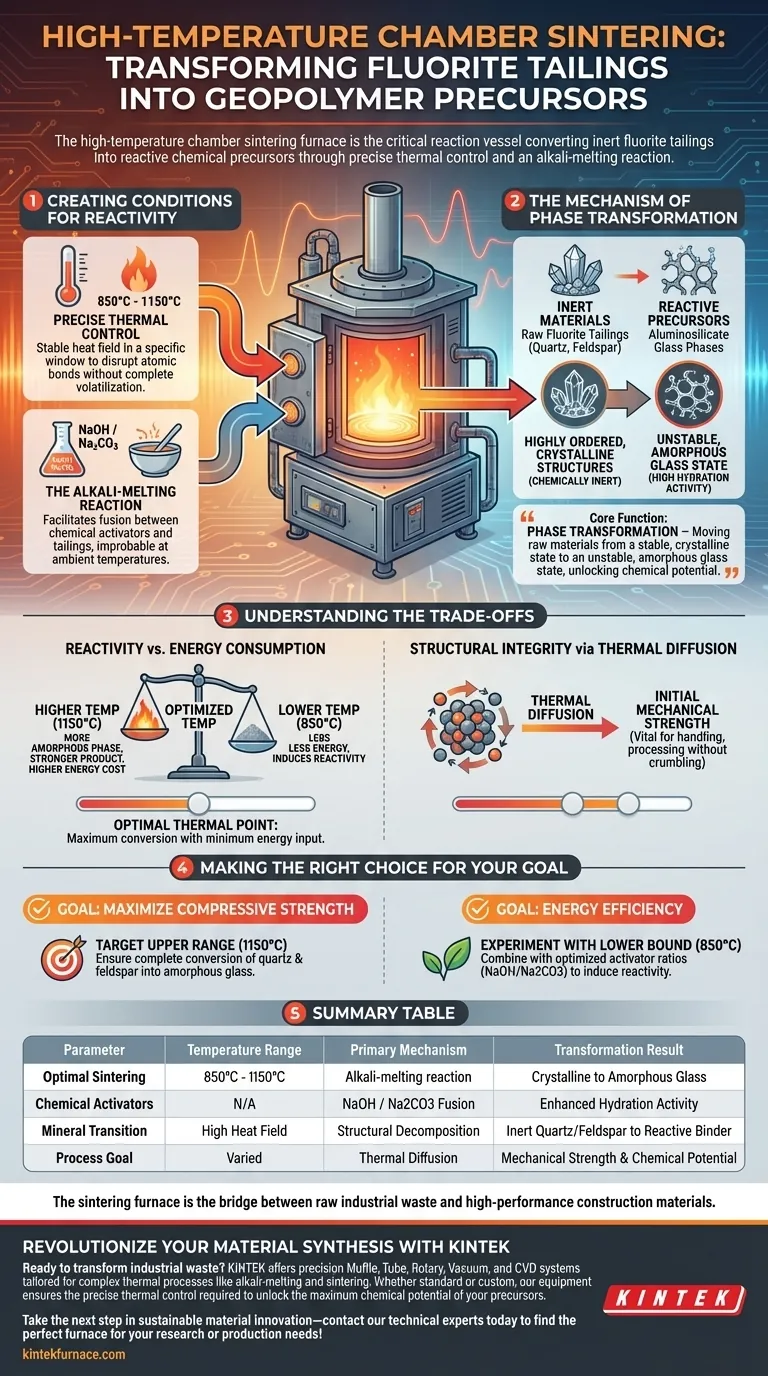
The high-temperature chamber sintering furnace serves as the critical reaction vessel that converts inert fluorite tailings into reactive chemical precursors. By maintaining a precisely controlled thermal environment between 850°C and 1150°C, the furnace drives an alkali-melting reaction that breaks down stable mineral structures into active forms capable of hydration.
The core function of this furnace is phase transformation. It moves the raw materials from a stable, crystalline state to an unstable, amorphous glass state, effectively unlocking the chemical potential necessary for geopolymerization.

Creating the Conditions for Reactivity
To turn waste tailings into useful binders, you must overcome the material's natural stability. The sintering furnace achieves this through specific thermal and chemical mechanisms.
Precise Thermal Control
The furnace generates a stable heat field within a specific window: 850°C to 1150°C. This range is critical because it provides enough energy to disrupt atomic bonds without completely volatilizing the necessary chemical components.
The Alkali-Melting Reaction
Inside the chamber, the fluorite tailings are treated with chemical activators, typically NaOH or Na2CO3. The furnace facilitates a fusion reaction between these activators and the tailings, a process that would not occur effectively at ambient temperatures.
The Mechanism of Phase Transformation
The most significant contribution of the sintering furnace is the alteration of the material's mineralogy. This is the difference between a filler material and a reactive binder.
Decomposing Crystalline Phases
Raw fluorite tailings are composed largely of quartz and feldspar. These naturally occurring minerals possess highly ordered, crystalline structures that are chemically inert. Without thermal treatment, they provide little to no binding strength.
Generating Amorphous Glass Phases
The heat of the furnace causes these crystalline structures to collapse. As they decompose, they transform into amorphous aluminosilicate glass phases.
Unlocking Hydration Activity
This transition to an "amorphous" (disordered) state is the key to utility. The disordered atomic structure is chemically unstable, meaning it has high hydration activity. When the resulting precursor is later mixed with water, it reacts vigorously to form the geopolymer network.
Understanding the Trade-offs
While the primary goal is chemical activation, the sintering process involves physical and operational considerations that must be balanced.
Reactivity vs. Energy Consumption
Higher temperatures (closer to 1150°C) generally yield a higher percentage of the amorphous glass phase, leading to a stronger final product. However, this increases energy costs significantly. You must find the optimal thermal point where maximum conversion occurs with minimum energy input.
Structural Integrity via Thermal Diffusion
Beyond chemical changes, the furnace facilitates thermal diffusion between particles. This imparts initial mechanical strength to the precursor bodies. This structural integrity is vital, ensuring the material is robust enough to handle during subsequent processing or transport without crumbling back into dust.
Making the Right Choice for Your Goal
When configuring your sintering process for fluorite tailings, your operational parameters should be dictated by your specific end-product requirements.
- If your primary focus is maximizing compressive strength: Target the upper temperature range (1150°C) to ensure the complete conversion of quartz and feldspar into amorphous aluminosilicate glass.
- If your primary focus is energy efficiency: Experiment with the lower temperature bound (850°C) combined with optimized activator ratios (NaOH/Na2CO3) to induce reactivity without excessive heat load.
The sintering furnace is the bridge between raw industrial waste and high-performance construction materials.
Summary Table:
| Parameter | Temperature Range | Primary Mechanism | Transformation Result |
|---|---|---|---|
| Optimal Sintering | 850°C - 1150°C | Alkali-melting reaction | Crystalline to Amorphous Glass |
| Chemical Activators | N/A | NaOH / Na2CO3 Fusion | Enhanced Hydration Activity |
| Mineral Transition | High Heat Field | Structural Decomposition | Inert Quartz/Feldspar to Reactive Binder |
| Process Goal | Varied | Thermal Diffusion | Mechanical Strength & Chemical Potential |
Revolutionize Your Material Synthesis with KINTEK
Ready to transform industrial waste into high-performance geopolymer binders? Backed by expert R&D and manufacturing, KINTEK offers precision Muffle, Tube, Rotary, Vacuum, and CVD systems tailored for complex thermal processes like alkali-melting and sintering. Whether you need a standard laboratory setup or a fully customizable high-temperature furnace, our equipment ensures the precise thermal control required to unlock the maximum chemical potential of your precursors.
Take the next step in sustainable material innovation—contact our technical experts today to find the perfect furnace for your research or production needs!
Visual Guide
References
- Hao Qiu, Xiao Wang. Preparation and mechanical performance of fluorite tailings geopolymer precursor under alkaline heat activation. DOI: 10.1038/s41598-024-82560-y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- Why is temperature range important when selecting a muffle furnace? Ensure Process Success and Equipment Longevity
- What are the key features of muffle furnace construction? Discover Precision and Safety in High-Temp Labs
- Why are drying ovens and calcination furnaces required for AuNPs on STFO? Optimize Your Catalyst Activation
- Why is a high-temperature blackbody furnace required for calibration? Ensure Precision in Tuyere Flame Measurement
- What role does a laboratory high-temperature muffle furnace play in the preparation of g-C3N4? Optimize Synthesis Now
- How does an elevator high-temperature furnace ensure process efficiency? Optimize S53P4 Bioactive Glass Production
- How does a laboratory muffle furnace facilitate the biomass carbonization process? Achieve Precise Biochar Production
- What are the main applications of a muffle furnace? Unlock Precision Heating for Material Transformations



















