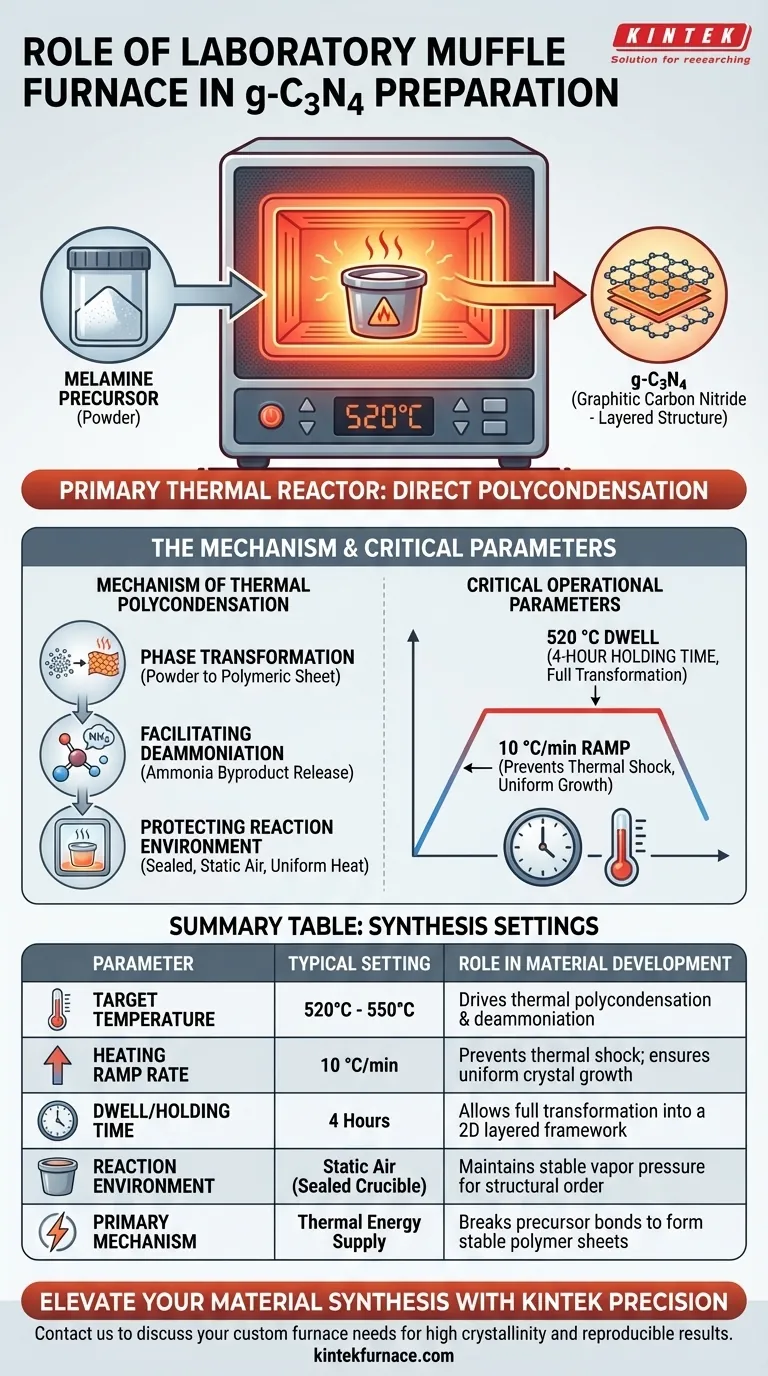
A laboratory high-temperature muffle furnace acts as the primary thermal reactor necessary for converting melamine precursors into graphitic carbon nitride (g-C3N4). It facilitates direct polycondensation by executing a precise heating program—typically ramping to 520 °C—to drive the chemical transformation from simple monomers into a stable, high-crystallinity polymerized structure.
Core Takeaway The muffle furnace does more than simply heat the material; it provides a stable, static environment critical for controlling reaction kinetics. By maintaining a specific heating rate and dwell time, the furnace ensures the complete deammoniation and condensation required to form a regular, two-dimensional layered carbon nitride framework.

The Mechanism of Thermal Polycondensation
Driving Phase Transformation
The fundamental role of the muffle furnace is to supply the energy required to break the chemical bonds of the precursor (melamine) and form new ones.
This process, known as thermal polycondensation, converts the monomer powder into a polymeric sheet.
Facilitating Deammoniation
During synthesis, the material must undergo deammoniation, where ammonia is released as a byproduct.
The furnace maintains the necessary temperature (often between 520 °C and 550 °C) to ensure these condensation reactions reach completion, preventing incomplete polymerization.
Protecting the Reaction Environment
The primary reference notes that this process often occurs within a sealed crucible placed inside the furnace.
The muffle furnace heats this enclosed environment evenly, allowing the material to polymerize under "static air" conditions, which favors the formation of the desired 2D layered structure.
Critical Operational Parameters
Precise Heating Ramps
The rate at which the temperature rises is as important as the final temperature itself.
A controlled ramp rate, specifically 10 °C/min, is essential to guide the smooth thermal polycondensation of molecules.
This gradual heating prevents thermal shock and allows the crystalline structure to develop uniformly.
Sustained Temperature Dwell
Once the target temperature (e.g., 520 °C) is reached, the furnace must hold this heat without fluctuation.
A standard protocol involves a 4-hour holding time, which provides sufficient duration for the precursors to fully transform into a highly crystalline graphitic network.
Understanding the Trade-offs
Static vs. Dynamic Atmospheres
Muffle furnaces typically operate with a static air environment, which is suitable and often preferred for standard g-C3N4 synthesis in crucibles.
However, they generally lack the sophisticated gas flow controls found in tube furnaces.
Uniformity Limits
While effective for batch synthesis in crucibles, muffle furnaces must be loaded carefully.
Overcrowding the chamber can lead to slight thermal gradients, potentially resulting in uneven crystallinity across different batches of the material.
Making the Right Choice for Your Goal
To optimize the preparation of graphitic carbon nitride, align your furnace settings with your specific structural requirements.
- If your primary focus is high crystallinity: Ensure your furnace is programmed for a steady 10 °C/min ramp to 520 °C, holding strictly for 4 hours to maximize structural order.
- If your primary focus is reproducible batch synthesis: Use a semi-closed system (sealed crucible) within the muffle furnace to maintain a stable vapor pressure of the precursor during heating.
Success in g-C3N4 synthesis relies not just on reaching high temperatures, but on the precise control of the thermal journey provided by the furnace.
Summary Table:
| Parameter | Typical Setting for g-C3N4 Synthesis | Role in Material Development |
|---|---|---|
| Target Temperature | 520 °C - 550 °C | Drives thermal polycondensation and deammoniation |
| Heating Ramp Rate | 10 °C/min | Prevents thermal shock; ensures uniform crystal growth |
| Dwell/Holding Time | 4 Hours | Allows full transformation into a 2D layered framework |
| Reaction Environment | Static Air (Sealed Crucible) | Maintains stable vapor pressure for structural order |
| Primary Mechanism | Thermal Energy Supply | Breaks precursor bonds to form stable polymer sheets |
Elevate Your Material Synthesis with KINTEK Precision
High-performance g-C3N4 synthesis requires more than just heat; it demands the absolute thermal stability and precise ramp control found in KINTEK’s laboratory high-temperature furnaces. Backed by expert R&D and world-class manufacturing, we provide a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to meet your specific research or production requirements.
Whether you are focusing on high crystallinity or reproducible batch synthesis, KINTEK offers the specialized equipment needed to master your material's thermal journey. Contact us today to discuss your custom furnace needs and see how our advanced heating solutions can empower your next breakthrough.
Visual Guide
References
- Yongjun Liu, Zhiming Huang. Photocatalytic reduction of aqueous chromium(<scp>vi</scp>) by RuO<sub>2</sub>/g-C<sub>3</sub>N<sub>4</sub> composite under visible light irradiation. DOI: 10.1039/d5ra00883b
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What safety advantages do electric furnaces offer? Eliminate Combustion Risks for a Safer Lab
- How does the digital interface of a muffle furnace enhance its functionality? Unlock Precision and Efficiency in Your Lab
- What role do box type resistance furnaces play in semiconductor processes? Essential for Thermal Oxidation and Annealing
- Why is an industrial muffle furnace required for Zirconia supports? Engineering High-Performance Catalyst Platforms
- How is a high-temperature muffle furnace utilized to determine the ash content of asphalt samples? Guide to Lab Success
- What should be done if the silicon carbon rod in the muffle furnace's resistance furnace ages or underperforms? Expert Tips for Optimal Performance
- What role does a laboratory high-temperature furnace play during the pyrolysis stage of UHTCMCs?
- What experimental conditions does a programmable muffle furnace provide for fire-retardant coatings? Lab Precision



















