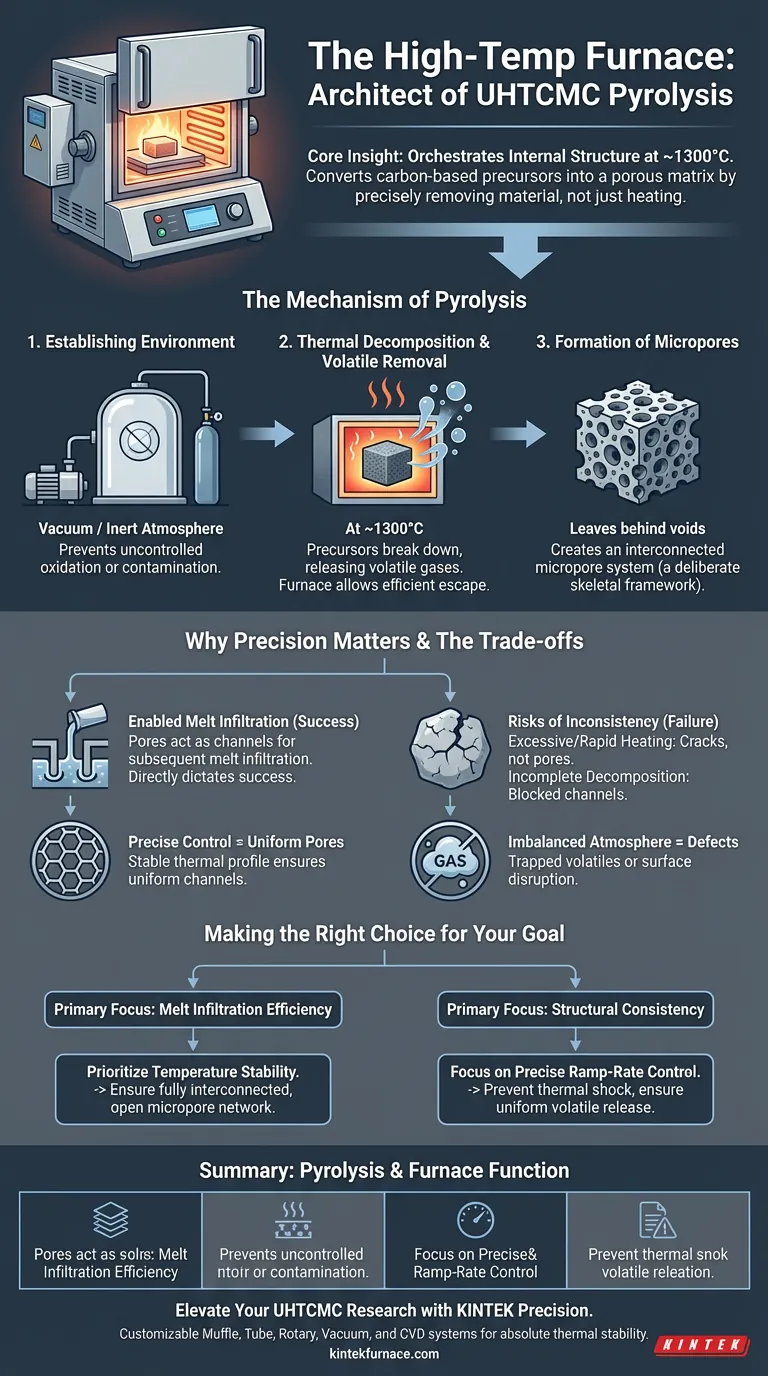
A laboratory high-temperature furnace acts as the primary architect of the composite's internal microstructure during pyrolysis. Specifically, it creates a controlled environment—typically at 1300°C—to convert carbon-based precursors into a porous matrix, establishing the necessary framework for subsequent processing steps.
Core Insight: The furnace’s role extends beyond simple heating; it orchestrates the precise removal of material. By controlling thermal decomposition, the furnace "hollows out" specific pathways within the matrix, creating an interconnected pore system essential for the composite's final density and strength.
The Mechanism of Pyrolysis
Establishing the Environment
The furnace must provide a stable vacuum or inert atmosphere throughout the process.
This isolation is critical. It prevents uncontrolled oxidation or contamination from the outside air while the material undergoes chemical transformation.
Thermal Decomposition and Volatile Removal
At temperatures approximating 1300°C, the furnace initiates the thermal decomposition of carbon-based precursors within the preform.
As these precursors break down, they release volatile gases. The furnace allows these volatiles to escape efficiently, physically removing mass from the composite structure.
Formation of Micropores
The evacuation of volatiles leaves behind voids, resulting in the formation of an interconnected micropore system.
This is not a defect, but a deliberate feature. The furnace transforms a dense precursor into a porous skeleton, which is the defining characteristic of a successful pyrolysis stage.
Why Furnace Precision Matters
Enabling Melt Infiltration
The micropores created during pyrolysis serve a functional purpose: they act as channels for melt infiltration.
If the furnace creates a closed or disconnected pore structure, the molten material in the next stage cannot penetrate the matrix. The quality of the pyrolysis directly dictates the success of the infiltration.
Controlling Pore Distribution
The precision of the temperature control directly determines the final porosity and the distribution of pores.
Fluctuations in temperature can lead to uneven pore sizes or localized density variations. A stable thermal profile ensures the "channels" are uniform throughout the composite.
Understanding the Trade-offs
The Risk of Thermal Inconsistency
While high heat is necessary, excessive or rapid heating can be detrimental.
If the temperature ramps up too quickly, volatiles may expand explosively, causing cracks rather than micropores. If the temperature is too low, decomposition remains incomplete, blocking the channels needed for infiltration.
Balancing Atmosphere and Pressure
Maintaining the correct vacuum or inert pressure is a delicate balance.
Insufficient vacuum can trap volatiles inside the matrix, leading to bloating or structural defects. However, overly aggressive vacuum conditions might disrupt the surface integrity of the preform.
Making the Right Choice for Your Goal
To ensure the structural integrity of Ultra-High Temperature Ceramic Matrix Composites, your approach to furnace operation should align with your specific processing targets:
- If your primary focus is Melt Infiltration Efficiency: Prioritize temperature stability to ensure the formation of a fully interconnected, open micropore network.
- If your primary focus is Structural Consistency: Focus on precise ramp-rate control to prevent thermal shock and ensure uniform volatile release across the entire geometry.
Ultimately, the laboratory furnace is not just a heat source; it is the tool that defines the permeability and future strength of your composite material.
Summary Table:
| Pyrolysis Phase | Furnace Function | Outcome for UHTCMC |
|---|---|---|
| Atmosphere Control | Provides vacuum/inert isolation | Prevents oxidation and contamination |
| Thermal Decomposition | Controlled heating at ~1300°C | Removes volatiles from carbon precursors |
| Microstructure Design | Precision ramp-rate control | Creates interconnected micropore networks |
| Process Integration | Channel formation | Enables successful subsequent melt infiltration |
Elevate Your UHTCMC Research with KINTEK Precision
The integrity of your ceramic matrix composites depends on the precision of your pyrolysis environment. KINTEK provides industry-leading thermal solutions designed specifically for the rigorous demands of advanced materials research.
Backed by expert R&D and manufacturing, KINTEK offers customizable Muffle, Tube, Rotary, Vacuum, and CVD systems that ensure absolute thermal stability and atmosphere control. Whether you are optimizing melt infiltration efficiency or structural consistency, our lab high-temp furnaces deliver the uniform heating required to define your material’s future strength.
Ready to master your pyrolysis stage? Contact KINTEK today for a customized furnace solution.
Visual Guide
References
- Luis Baier, Vito Leisner. Development of ultra-high temperature ceramic matrix composites for hypersonic applications via reactive melt infiltration and mechanical testing under high temperature. DOI: 10.1007/s12567-024-00562-y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- What is the function of a high-temperature muffle furnace? Master Eggshell Adsorbent Activation
- How does the furnace atmosphere contribute to the function of a muffle furnace? Unlock Precision in Material Processing
- What are the differences in insulation between muffle furnaces and drying ovens? Key Design Insights for Your Lab
- Why is a muffle furnace essential for Sn:ZnO nanopowders? Achieve Perfect Crystal Structure and Purity
- What atmosphere control options are available in advanced muffle furnaces? Master Materials Processing with Precision
- How does a high-temperature muffle furnace ensure precision during phase transformation research of manganese ore?
- What role does a high-temperature muffle furnace play in kaolin pretreatment? Achieve Perfect Metakaolin Activation
- What is the structure of a box type electric furnace? Uncover the Core Components for Precise Heating



















