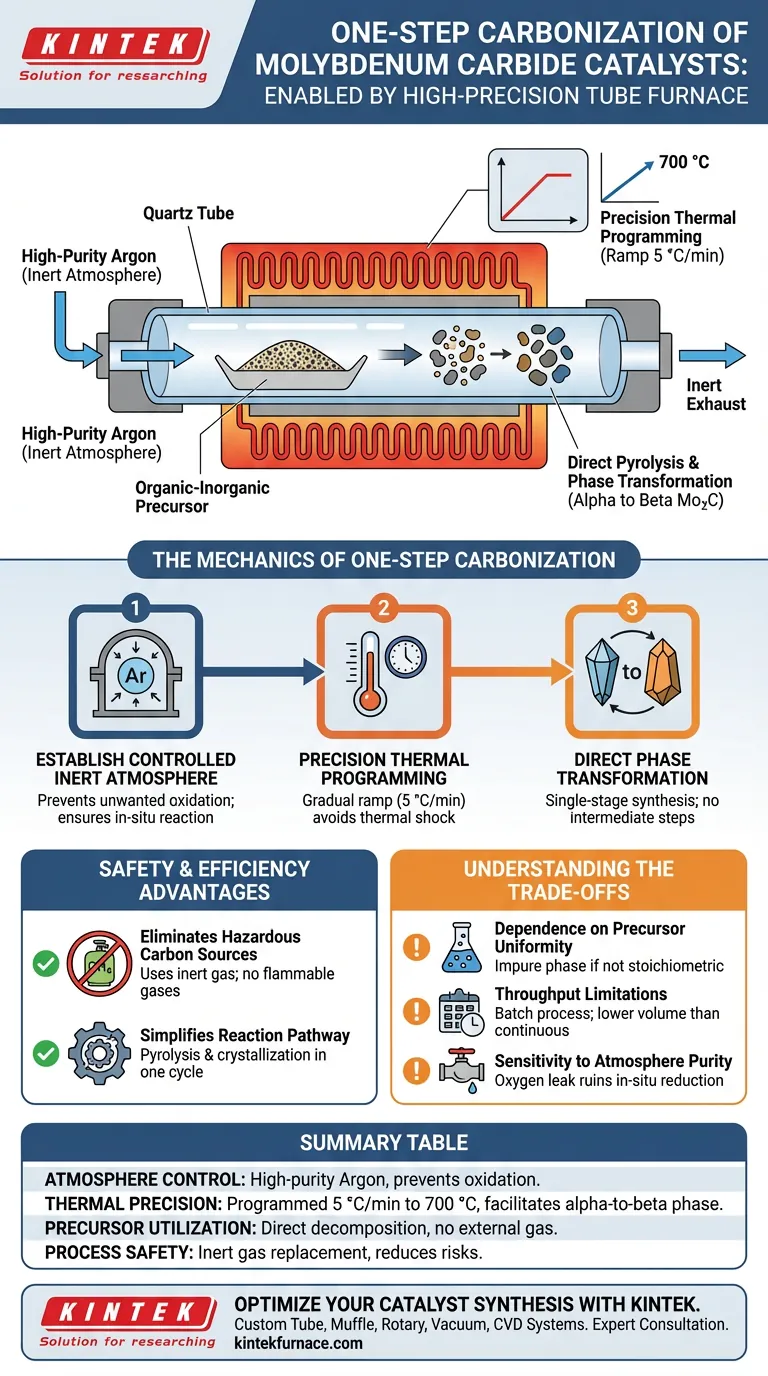
A high-precision tube furnace facilitates the one-step carbonization of molybdenum carbide by creating a sealed, inert environment that allows for the direct pyrolysis of organic-inorganic hybrid precursors. Instead of introducing external carbon gases, the furnace utilizes a high-purity argon atmosphere and a programmed heating ramp to 700 °C, driving the in-situ transformation of the precursor material into the desired catalytic phase.
By leveraging an inert atmosphere rather than reactive hydrocarbon gases, the high-precision tube furnace enables a safer, single-stage synthesis. It controls the thermal decomposition of hybrid precursors to achieve a direct phase transition from alpha to beta molybdenum carbide.

The Mechanics of One-Step Carbonization
Establishing a Controlled Inert Atmosphere
The fundamental requirement for this one-step method is the exclusion of oxygen and reactive gases. The tube furnace provides a sealed environment maintained under a flow of high-purity argon.
This inert atmosphere prevents unwanted oxidation during the heating process. It ensures that the chemical reactions occur solely between the components of the precursor material, known as in-situ carbonization.
Precision Thermal Programming
Success in this method relies on a strictly controlled heating rate, specifically a programmed rise of 5 °C/min. The furnace steadily increases the temperature to a target of 700 °C.
This gradual ramp allows for the orderly decomposition of the organic components within the precursor. Precise temperature control helps avoid thermal shock or rapid volatilization, which could disrupt the formation of the catalyst structure.
Direct Phase Transformation
Under these specific thermal and atmospheric conditions, the organic-inorganic hybrid precursor undergoes direct pyrolysis. The furnace facilitates a crystalline phase transformation, specifically converting the material from the alpha phase to the beta phase of molybdenum carbide.
This eliminates the need for intermediate steps or separate reduction processes often required in traditional synthesis methods.
Safety and Efficiency Advantages
Eliminating Hazardous Carbon Sources
Traditional carbonization often requires the introduction of flammable or explosive gases, such as methane, to provide the carbon source.
The one-step method supported by the tube furnace avoids these safety risks entirely. Because the carbon is derived directly from the organic portion of the solid precursor, only inert argon gas is required for operation.
Simplifying the Reaction Pathway
By combining pyrolysis and crystallization into a single operation, the furnace streamlines the production workflow. The "one-step" nature of the process means that reduction and carbonization happen simultaneously within the same thermal cycle.
Understanding the Trade-offs
Dependence on Precursor Uniformity
While the furnace controls the environment, the chemistry relies heavily on the "organic-inorganic hybrid precursor." If the precursor is not perfectly mixed or stoichiometric (e.g., the ratio of the carbon source to the molybdenum source), the furnace cannot correct for it, leading to impure phases.
Throughput Limitations
High-precision tube furnaces operate as batch reactors. While excellent for achieving specific crystalline phases (alpha vs. beta) and high purity, they generally offer lower throughput compared to continuous industrial processes like rotary kilns.
Sensitivity to Atmosphere Purity
The "high-precision" aspect extends to the gas seal. Even a minor leak introducing oxygen can ruin the in-situ reduction process. The method is entirely dependent on the integrity of the argon environment; unlike reducing atmospheres (H2), argon cannot actively "scrub" oxygen that leaks into the system.
Making the Right Choice for Your Goal
To maximize the effectiveness of your molybdenum carbide preparation, consider your primary objectives:
- If your primary focus is Safety and Simplicity: Utilize this one-step method with high-purity argon to eliminate the infrastructure and risks associated with handling explosive gases like methane.
- If your primary focus is Phase Purity: Adhere strictly to the 5 °C/min ramp rate to 700 °C, as deviations in thermal history can fail to trigger the specific alpha-to-beta phase transition.
- If your primary focus is Material Consistency: Ensure the organic-inorganic precursor is chemically homogeneous before loading, as the furnace fixes the structure in situ based on the initial mixing.
The high-precision tube furnace ultimately acts as a stabilizer, allowing complex chemical transformations to occur safely through rigorous environmental control rather than complex chemical inputs.
Summary Table:
| Feature | Mechanism in One-Step Carbonization | Benefit |
|---|---|---|
| Atmosphere Control | High-purity Argon flow in a sealed tube | Prevents oxidation; enables in-situ pyrolysis |
| Thermal Precision | Programmed 5 °C/min ramp to 700 °C | Facilitates alpha to beta phase transformation |
| Precursor Utilization | Direct decomposition of organic-inorganic hybrids | Eliminates the need for external hydrocarbon gases |
| Process Safety | Replacement of methane with inert gas | Reduces explosion risks and simplifies infrastructure |
Optimize Your Catalyst Synthesis with KINTEK
Unlock the full potential of your research with KINTEK’s high-precision thermal solutions. Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique laboratory needs.
Whether you are performing complex in-situ carbonization or high-purity material sintering, our furnaces provide the stability and atmospheric integrity required for consistent results. Our technical team is ready to help you select or design the ideal system for your specific application.
Ready to enhance your lab's efficiency and safety?
Contact KINTEK Today for a Expert Consultation
Visual Guide
References
- Linyuan Zhou, Changwei Hu. Regulating the Hydrodeoxygenation Activity of Molybdenum Carbide with Different Diamines as Carbon Sources. DOI: 10.3390/catal14020138
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- What are the common applications of a horizontal tube furnace? Unlock Precision in High-Temperature Processing
- How is the structure of a multi station vacuum tube furnace divided? Optimize Your Lab's Thermal Processing
- What is a high temperature tube furnace? Achieve Precise Heat and Atmosphere Control
- What are the primary applications of lab tubular furnaces in material science and engineering? Precision Heat for Advanced Materials
- Why are quartz or alumina tubes used in tube furnaces? Key Benefits for High-Temp Processes
- What is the importance of segmented temperature control in a tube furnace for Cu/Zn-SAN? Master Atomic Dispersion
- What are the typical physical and performance specifications for lab tube furnaces? A Guide to Key Specs
- What is the necessity of annealing treatment for CuCo2O4@rGO? Optimize High-Crystallinity Synthesis in Tube Furnaces



















