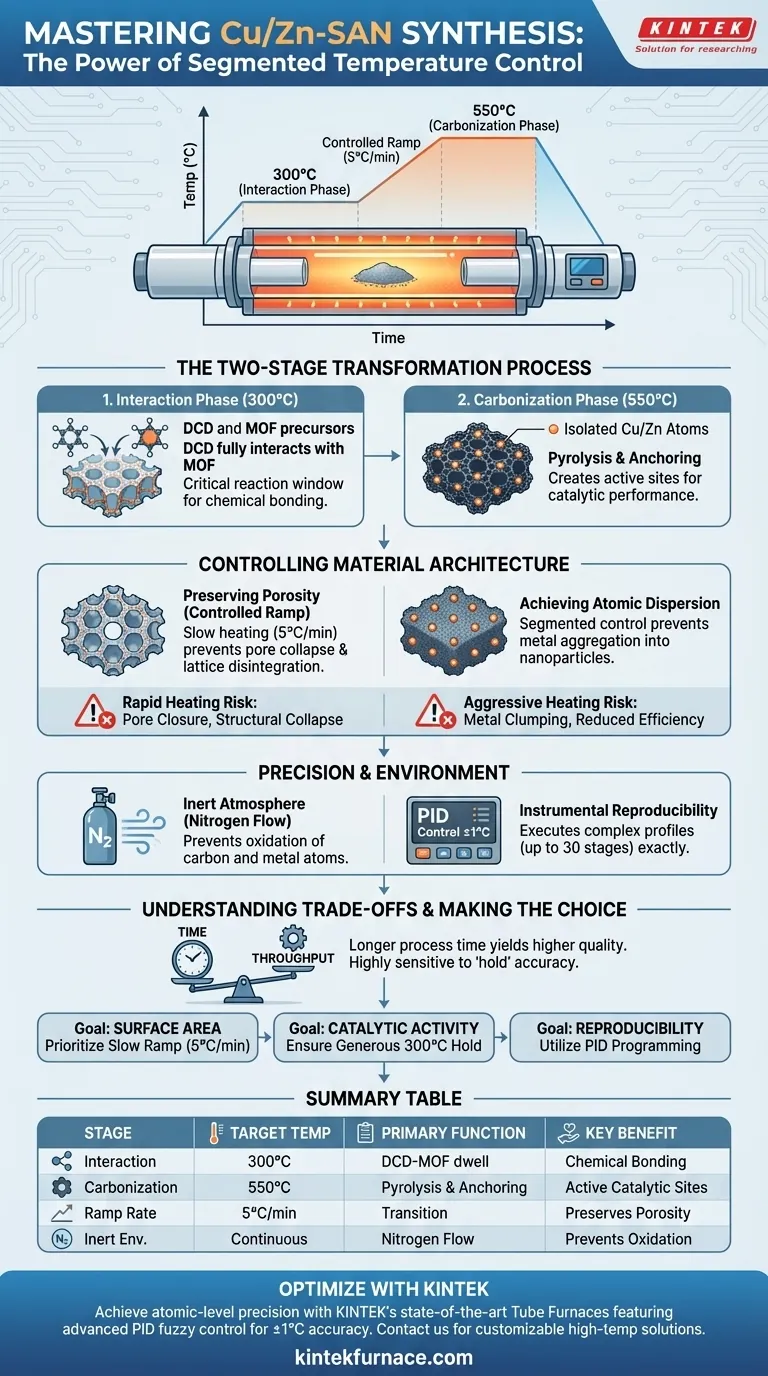
Segmented temperature control is the fundamental mechanism that enables the distinct chemical phases required to synthesize high-quality Cu/Zn-SAN. By programming precise hold times—specifically an initial dwell at 300°C followed by a ramp to 550°C—you separate the precursor interaction phase from the final carbonization phase. This separation is vital for achieving atomic-level dispersion and preserving the structural integrity of the material.
Core Insight Without staged heating, the synthesis process risks structural collapse and the formation of unwanted metal clusters. Segmented control facilitates a necessary two-step evolution: allowing dicyandiamide (DCD) to fully interact with the Metal-Organic Framework (MOF) at lower temperatures, before locking in the structure through carbonization at higher temperatures.

The Two-Stage Transformation Process
The Interaction Phase (300°C)
The first critical segment involves holding the temperature at 300°C. This is not merely a warm-up; it is a reaction window.
During this dwell time, the dicyandiamide (DCD) must fully interact with the Metal-Organic Framework (MOF) precursors. If the temperature rises too quickly past this point, this chemical interaction remains incomplete, compromising the final material composition.
The Carbonization Phase (550°C)
Once the precursors have interacted, the temperature is ramped to 550°C to initiate pyrolysis. This stage converts the precursors into a nitrogen-doped carbon framework.
It is during this higher-temperature phase that the metal atoms (Copper and Zinc) are physically anchored into the structure. This creates the active sites required for the material's catalytic performance.
Controlling Material Architecture
Preserving Material Porosity
The specific heating rate used between segments, such as 5°C per minute, is just as important as the target temperatures.
A controlled, moderate ramp rate prevents the structural collapse of the MOF. Rapid heating can cause the pores to close up or the lattice to disintegrate, destroying the surface area required for effective catalysis.
Achieving Atomic Dispersion
The ultimate goal of Cu/Zn-SAN synthesis is to keep metal atoms isolated rather than letting them clump together.
Segmented control prevents metal atom aggregation. If the thermal energy is applied too aggressively, the Copper and Zinc atoms will migrate and fuse into nanoparticles, significantly reducing the material's efficiency.
The Role of Precision and Environment
The Necessity of an Inert Atmosphere
While temperature segments drive the reaction, the environment protects it. The tube furnace must utilize flowing nitrogen to create a strictly inert atmosphere.
This prevents the oxidation of both the carbon substrate and the metal atoms during the sensitive 300°C to 550°C window.
Instrumental Reproducibility
Modern tube furnaces utilize PID self-learning fuzzy control systems to maintain temperatures within ±1°C.
This precision is required to execute complex heating curves (up to 30 independent stages). It ensures that the specific interaction and anchoring phases occur exactly as designed, experiment after experiment.
Understanding the Trade-offs
Time vs. Throughput
Segmented temperature control significantly extends the duration of the synthesis process. The requirement for slow ramp rates (e.g., 5°C/min) and long holding times means lower throughput compared to rapid firing methods.
Sensitivity to Deviation
The process is highly sensitive to the accuracy of the "hold" segments. If the furnace overshoots the 300°C mark or fails to hold it long enough, the DCD-MOF interaction will be insufficient, rendering the subsequent carbonization step less effective.
Making the Right Choice for Your Goal
To maximize the quality of your Cu/Zn-SAN preparation, tailor your thermal profile to your specific structural needs:
- If your primary focus is Surface Area: Prioritize a slow, steady ramp rate (max 5°C/min) to prevent pore collapse during the transition to 550°C.
- If your primary focus is Catalytic Activity: Ensure the hold time at 300°C is generous to guarantee complete DCD-MOF interaction and maximal single-atom anchoring.
- If your primary focus is Reproducibility: Utilize the furnace's PID programming to lock in the exact segment profile, eliminating manual variability between batches.
Success in Cu/Zn-SAN synthesis relies not on how hot you get the furnace, but on how precisely you control the journey to those temperatures.
Summary Table:
| Synthesis Stage | Target Temp | Primary Function | Key Benefit |
|---|---|---|---|
| Interaction Phase | 300°C | DCD-MOF precursor dwell | Ensures chemical bonding |
| Carbonization Phase | 550°C | Pyrolysis & anchoring | Creates active catalytic sites |
| Ramp Rate (5°C/min) | Transition | Controlled lattice heating | Preserves material porosity |
| Inert Environment | Continuous | Nitrogen flow | Prevents metal oxidation |
Optimize Your Advanced Material Synthesis with KINTEK
Achieving atomic-level dispersion in Cu/Zn-SAN requires the extreme precision of professional-grade thermal equipment. KINTEK provides state-of-the-art Tube, Muffle, Rotary, and Vacuum systems, featuring advanced PID fuzzy control to manage up to 30 independent heating stages with ±1°C accuracy.
Whether you need customizable high-temp furnaces for CVD or specialized lab setups, our expert R&D and manufacturing teams are ready to support your unique research requirements. Contact KINTEK today to discover how our precision heating solutions can ensure the structural integrity and catalytic performance of your next breakthrough material.
Visual Guide
References
- Eslam M. Hamed, Sam Fong Yau Li. Bimetallic Cu/Zn Single‐Atom Nanozyme with Superoxide Dismutase‐Like Activity. DOI: 10.1002/smll.202503879
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What is the function of a high-temperature tube furnace in the synthesis of SPC-Fe? Master Graphitic Carbon Production
- What are the main components of a tube furnace? Essential Parts for Precise High-Temperature Processing
- What is the operational principle of a 70mm tube furnace? Master Precise Heat and Atmosphere Control
- What is a Tube Furnace? Master Precision Heating for Sensitive Materials
- What is the function of a Tube Furnace during molybdenum carbide synthesis? Master Catalyst Carbonization
- How does the configuration of a quartz inner tube benefit WTe2 CVD growth? Optimize Precision Thin Film Engineering
- What is sintering, and how is it performed in horizontal furnaces? Unlock Precision in Powder Processing
- How is a laboratory tube furnace utilized to convert metal-organic precursors? Master Thin Film Pyrolysis Today



















