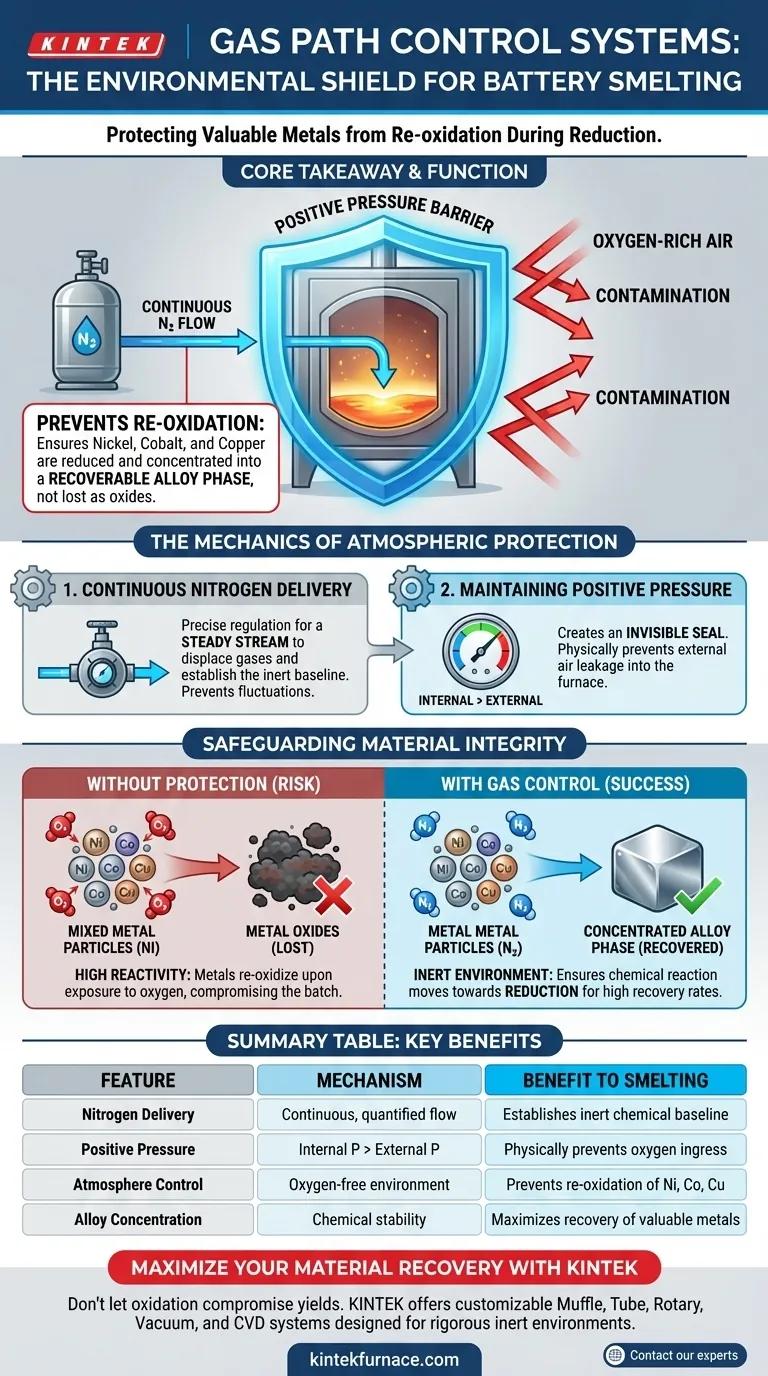
Gas path control systems act as a critical environmental shield. By strictly regulating the continuous flow of nitrogen, these systems generate a positive pressure barrier inside the furnace. This prevents oxygen-rich external air from entering the reaction chamber, ensuring the high-temperature environment remains inert to protect valuable metals from chemical degradation.
Core Takeaway: The system's primary function is to prevent the re-oxidation of transition metals like nickel, cobalt, and copper. By maintaining a pressurized, oxygen-free atmosphere, it ensures these metals are successfully reduced and concentrated into a recoverable alloy phase rather than being lost as oxides.

The Mechanics of Atmospheric Protection
Continuous Nitrogen Delivery
The foundation of the protection mechanism is the delivery of nitrogen to the reaction chamber. The system ensures this flow is both continuous and quantified.
This precise regulation prevents fluctuations in the furnace atmosphere. A steady stream is required to displace existing gases and establish the necessary chemical baseline for reduction.
Maintaining Positive Pressure
The physical mechanism of protection is positive pressure. By pumping nitrogen into the chamber, the system ensures the internal pressure is higher than the external atmospheric pressure.
This pressure differential acts as an invisible seal. It physically prevents external air from leaking into the furnace, which is the primary source of contamination during the smelting process.
Safeguarding Material Integrity
Preventing Re-oxidation
At high smelting temperatures, transition metals are highly reactive. Specifically, nickel, cobalt, and copper are susceptible to re-oxidizing if exposed to oxygen.
The gas path control system eliminates this risk by maintaining an inert or reducing environment. This atmosphere ensures that the chemical reaction moves in the desired direction—reduction—rather than reverting to oxidation.
Concentrating the Alloy Phase
The ultimate economic goal of recycling spent batteries is the recovery of valuable materials.
By preventing re-oxidation, the system ensures that the target metals settle into the alloy phase. This concentration is essential for high recovery rates and the successful extraction of reusable materials.
Understanding the Operational Risks
The Consequence of Flow Interruption
The protection provided by the system is active, not passive. It relies entirely on the continuous supply of nitrogen.
If the delivery system fails or fluctuates significantly, the positive pressure barrier collapses. This allows external air to ingress immediately, compromising the batch and oxidizing the metals.
Specificity of the Atmosphere
The system is designed for a specific chemical goal: reduction. It is not merely about keeping air out, but about maintaining a specific reducing potential.
Failure to quantify the nitrogen delivery correctly can lead to an unstable environment. This instability threatens the efficiency of the reduction process and the purity of the resulting alloy.
Making the Right Choice for Your Process
To maximize the efficiency of your reduction smelting operation, consider the following key objectives:
- If your primary focus is Recovery Yield: Ensure the control system is calibrated to maintain a strict positive pressure at all times to prevent the loss of nickel, cobalt, and copper to oxidation.
- If your primary focus is Process Stability: Prioritize a system that guarantees a continuous, quantified flow of nitrogen to eliminate atmospheric fluctuations within the chamber.
By effectively isolating the reaction chamber from the outside world, the gas path control system serves as the guarantor of your material recovery rates.
Summary Table:
| Feature | Mechanism | Benefit to Smelting |
|---|---|---|
| Nitrogen Delivery | Continuous, quantified flow | Establishes an inert chemical baseline for reduction |
| Positive Pressure | Internal pressure > External pressure | Physically prevents oxygen ingress and air leaks |
| Atmosphere Control | Oxygen-free environment | Prevents re-oxidation of Ni, Co, and Cu |
| Alloy Concentration | Chemical stability | Maximizes recovery rates of valuable transition metals |
Maximize Your Material Recovery with KINTEK
Don’t let oxidation compromise your recycling yields. KINTEK’s advanced gas path control systems provide the precise atmospheric protection required for high-efficiency battery smelting. Backed by expert R&D and manufacturing, we offer customizable Muffle, Tube, Rotary, Vacuum, and CVD systems designed to maintain the rigorous inert environments your lab or industrial process demands.
Ready to optimize your reduction smelting process? Contact our high-temperature furnace experts today to discover how our tailored solutions can enhance your recovery rates and material purity.
Visual Guide
References
- Chen Wang, Hongbin Ling. Extraction of Valuable Metals from Spent Li-Ion Batteries Combining Reduction Smelting and Chlorination. DOI: 10.3390/met15070732
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why is the enhancement of coke strength essential? Maximize Blast Furnace Efficiency & Stability
- What is the technical value of a Hydrogen Reduction-type Test Furnace in green steelmaking? Scale Sustainable Production
- What core role does a magnetron sputtering system play in CrSiN-Y PVD? Unlock High-Performance Coating Precision
- How does the electric arc furnace contribute to carbon neutrality? Decarbonizing Steel with EAF Technology
- What is the purpose of coating aluminum electrodes with Au80Pd20? Enhancing Precision in Nanoparticle Characterization
- What role does a high-temperature annealing furnace play in the preparation of AAO substrates? Enhance Pore Regularity
- Why is a laboratory blast drying oven necessary for preparing Reduced Graphene Oxide precursors? Ensure Powder Quality
- Why is a nitrogen protection system necessary for LPF resin synthesis? Ensure Purity in Lab Polymerization



















