A dual-zone tube furnace facilitates monolayer synthesis by creating two distinct thermal environments within a single reaction chamber, allowing for the independent management of precursor sublimation and substrate deposition. By separating the evaporation temperature of volatile elements (like sulfur) from the higher reaction temperatures required for metal oxides (like MoO3), this equipment enables the precise Chemical Vapor Deposition (CVD) or Chemical Vapor Transport (CVT) needed to grow high-quality two-dimensional materials.
Core Takeaway The critical advantage of a dual-zone furnace is the decoupling of precursor evaporation from crystal growth. This separation allows you to fine-tune the vaporization rate of reactants without altering the reaction kinetics at the substrate, ensuring the specific conditions required for single-crystal, monolayer formation.
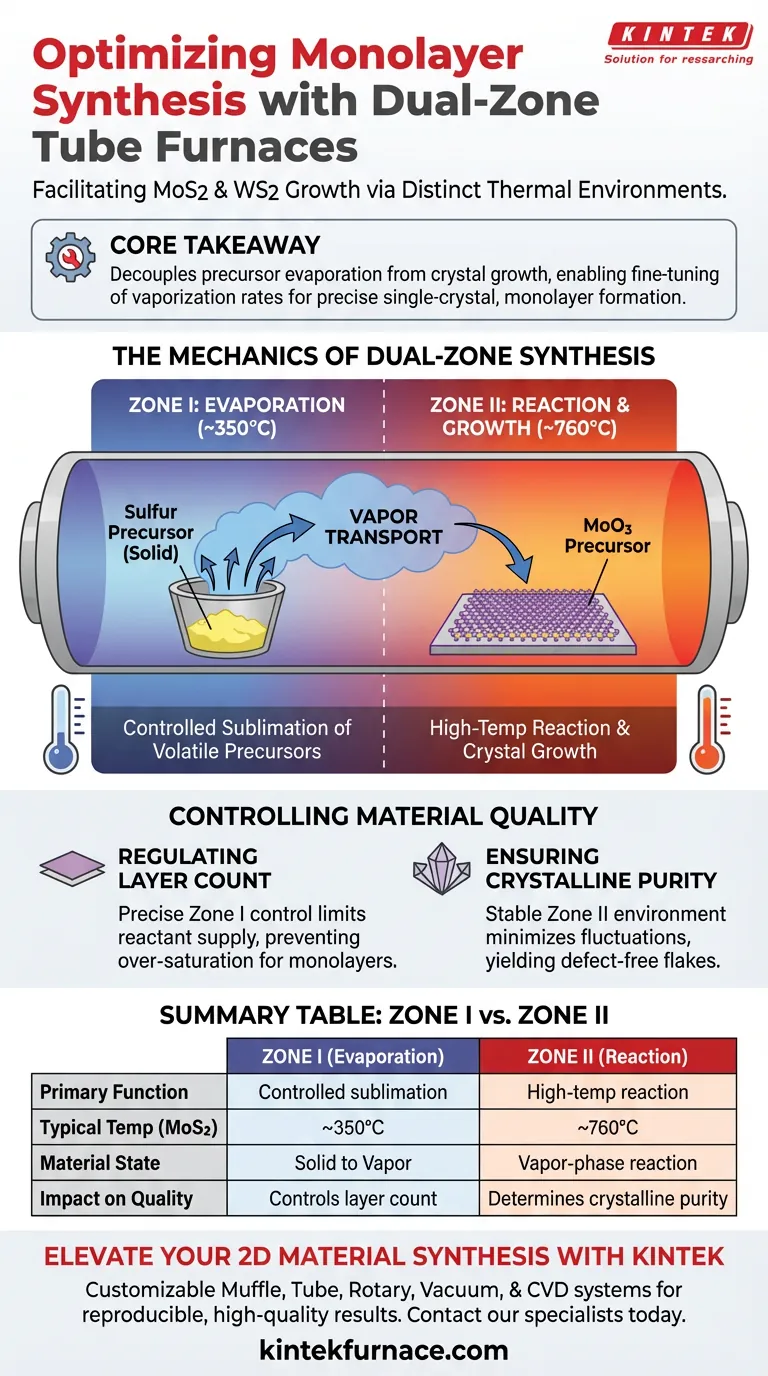
The Mechanics of Dual-Zone Synthesis
Independent Temperature Control
The defining feature of this furnace is its ability to maintain different temperatures in adjacent zones simultaneously.
For the synthesis of Molybdenum Disulfide (MoS2), the primary reference notes that Zone I may be set to 350°C while Zone II is heated to 760°C.
This independence allows the system to accommodate materials with vastly different physical properties within the same process flow.
Managing Precursor Volatility
Synthesis of transition metal dichalcogenides (TMDs) usually involves two precursors: a metal oxide (like MoO3) and a chalcogen (like Sulfur powder).
Sulfur is highly volatile and sublimates at relatively low temperatures. If exposed immediately to high heat, it would evaporate instantly, leading to an uncontrolled reaction.
The first lower-temperature zone ensures the sulfur sublimates at a controlled, steady rate before being transported downstream.
Controlled Vapor Transport
Once sublimated, the precursor vapors must move to the substrate to react.
The dual-zone setup creates a specific thermal gradient that drives the transport of these vapors.
The sulfur vapor travels from the cooler Zone I into the hotter Zone II, where it reacts with the metal oxide vapor and deposits onto the substrate.
Controlling Material Quality
Regulating Layer Count
The ultimate goal in this context is often to achieve a "monolayer"—a material only one molecule thick.
By precisely regulating the evaporation temperature in the first zone, you effectively control the "supply" of reactants.
This prevents the over-saturation of the substrate, allowing you to stop growth at a single layer rather than allowing bulk crystals to form.
Ensuring Crystalline Purity
A stable thermal environment is non-negotiable for high-quality electronics materials.
The tube furnace provides a uniform thermal environment that minimizes fluctuations during the growth phase.
This stability is essential for determining the crystalline quality and physical dimensions of the resulting MoS2 or WS2 flakes.
Understanding the Trade-offs
Sensitivity to Parameters
While dual-zone furnaces offer precision, they introduce complexity regarding process parameters.
The interaction between the two zones means that a slight deviation in the evaporation zone (Zone I) can drastically alter the stoichiometry in the reaction zone (Zone II).
Gradient Management
The transition area between the two temperature zones must be carefully considered.
If the thermal gradient is not managed correctly, precursors may condense prematurely between zones before reaching the target substrate.
Making the Right Choice for Your Goal
If you are setting up a synthesis protocol for 2D materials, consider how the furnace capabilities align with your specific objectives:
- If your primary focus is crystalline quality: Prioritize the precise regulation of Zone II (the reaction zone) to ensure a uniform thermal environment for defect-free flake growth.
- If your primary focus is controlling layer thickness: Focus on the independent control of Zone I (the evaporation zone) to strictly limit the supply rate of the volatile precursor (sulfur).
The dual-zone configuration effectively transforms the chaotic variable of vapor pressure into a tunable constant, making reproducible monolayer synthesis possible.
Summary Table:
| Feature | Zone I (Evaporation) | Zone II (Reaction) |
|---|---|---|
| Primary Function | Controlled sublimation of volatile precursors (e.g., Sulfur) | High-temp reaction and crystal growth (e.g., MoO3 + S) |
| Typical Temp (MoS2) | ~350°C | ~760°C |
| Material State | Solid to Vapor transition | Vapor-phase reaction & deposition |
| Impact on Quality | Controls layer count & supply rate | Determines crystalline purity & flake size |
Elevate Your 2D Material Synthesis with KINTEK
Precise thermal gradients are the secret to flawless monolayer growth. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your specific research demands. Whether you are synthesizing MoS2, WS2, or complex heterostructures, our dual-zone furnaces provide the independent temperature control and stability required for reproducible, high-quality results.
Ready to optimize your CVD process? Contact our laboratory specialists today to find the perfect furnace solution for your application.
Visual Guide
References
- Weihu Kong, Jie Ma. Excitonic Evolution in WS2/MoS2 van der Waals Heterostructures Turned by Out-of-Plane Localized Pressure. DOI: 10.3390/app14052179
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
People Also Ask
- Why is a continuous nitrogen flow required in a closed tube furnace during the solid-phase synthesis of LiMnO2 precursors?
- How do laboratory tube furnaces contribute to the sintering of Ba0.95La0.05(Fe1-xYx)O3-δ? Precise Atmosphere Control
- Why is a tube furnace considered essential for metal-zeolite catalysts? Unlock Porosity and Active Sites
- What are the physical characteristics of a graphite furnace used in atomic absorbance measurements? Uncover Its Design for Ultra-Trace Analysis
- What is the significance of using a tubular furnace in waste salt pyrolysis research? Precision for High-Fidelity Data
- What are the limitations of horizontal tube furnaces? Manage Space, Temperature, and Handling Challenges
- How does a horizontal tube furnace differ from a vertical tube furnace? Choose the Right Furnace for Your Lab
- How are horizontal furnaces utilized in the automotive sector? Boost Component Durability and Efficiency



















