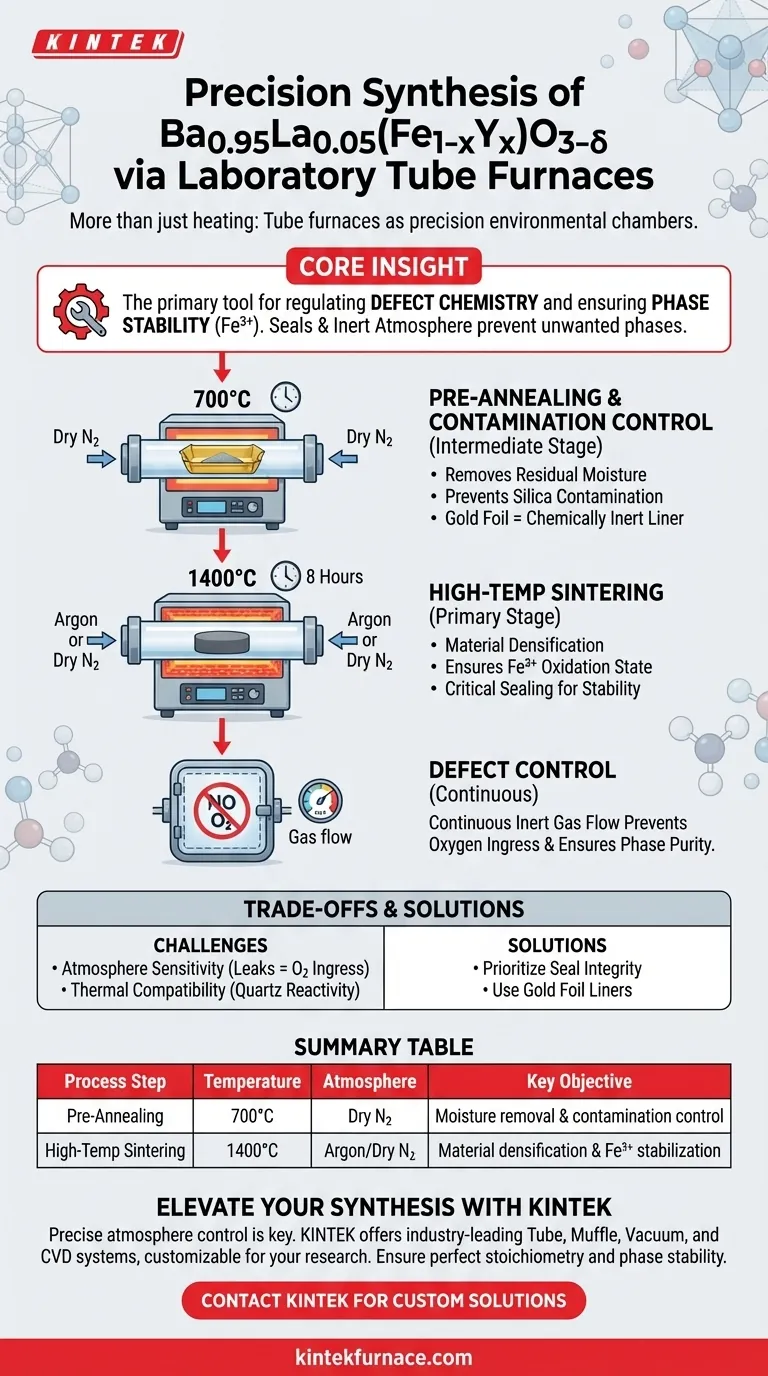
Laboratory tube furnaces serve as precision environmental chambers for the synthesis of Ba0.95La0.05(Fe1-xYx)O3-δ, going far beyond simple heating.
They facilitate critical thermal treatments by maintaining strictly controlled inert atmospheres, such as argon or dry nitrogen, during both the high-temperature sintering phase at 1400°C and the intermediate processing stage at 700°C. This specific environmental control is essential for stabilizing the chemical structure of the material.
Core Insight: The tube furnace is the primary tool for regulating defect chemistry and ensuring phase stability. Its ability to seal and hold an inert atmosphere ensures that iron remains in the crucial 3+ oxidation state, preventing the formation of unwanted mixed valences or secondary phases.

Achieving Phase Stability through Sintering
The synthesis of high-quality Ba0.95La0.05(Fe1-xYx)O3-δ relies heavily on the ability to control oxidation states during the sintering process.
The Role of High-Temperature Sintering
Sintering is typically conducted at 1400°C for approximately 8 hours.
At this temperature, the tube furnace ensures the consolidation of the material into a dense solid.
Controlling the Iron Oxidation State
The most critical function of the furnace during this stage is maintaining the iron elements entirely in the 3+ oxidation state.
To achieve this, the furnace operates under a flowing atmosphere of argon or dry nitrogen.
The precise sealing capabilities of the tube furnace prevent oxygen ingress, which is vital for obtaining a stable trivalent iron perovskite phase.
Pre-Annealing and Contamination Control
Before or after the primary sintering, intermediate thermal treatments at lower temperatures (around 700°C) are often required to refine the material's purity.
Eliminating Residual Moisture
Processing the sample at 700°C in a dry nitrogen environment effectively removes residual moisture.
This step ensures that hydrogen or water vapor does not interfere with the defect chemistry of the final oxide.
Preventing Reaction with the Vessel
During these stages, the sample is often placed within gold foil containers inside the high-purity quartz tube.
The gold foil acts as a chemically inert liner.
This is necessary because direct contact between the Ba0.95La0.05(Fe1-xYx)O3-δ sample and the quartz walls at high temperatures can lead to unwanted chemical reactions and silica contamination.
Understanding the Trade-offs
While tube furnaces offer precision, successful processing requires navigating specific limitations and risks.
Atmosphere Sensitivity
The process is highly sensitive to the integrity of the inert atmosphere.
Even minor leaks in the furnace seals can introduce oxygen, altering the defect chemistry and shifting the iron oxidation state away from the target 3+ valency.
Thermal Compatibility Materials
Choosing the right containment materials is a strict requirement, not an option.
Using standard crucibles or placing samples directly on the quartz tube can lead to irreversible contamination, ruining the sample's stoichiometry. The use of gold foil is a specific counter-measure to this trade-off.
Making the Right Choice for Your Goal
To ensure the successful synthesis of Ba0.95La0.05(Fe1-xYx)O3-δ, align your furnace protocols with your specific purity requirements.
- If your primary focus is Phase Purity (Iron 3+ Stability): Prioritize the integrity of the gas flow system and seals to maintain a strict Argon or Nitrogen atmosphere at 1400°C.
- If your primary focus is Compositional Accuracy: Ensure the use of gold foil liners during the 700°C step to prevent quartz contamination and reactivity.
Precision in the atmosphere and containment is just as critical as the temperature itself for stabilizing complex perovskites.
Summary Table:
| Process Step | Temperature | Atmosphere | Duration | Key Objective |
|---|---|---|---|---|
| Pre-Annealing | 700°C | Dry Nitrogen | Variable | Moisture removal & contamination control |
| High-Temp Sintering | 1400°C | Argon/Dry Nitrogen | 8 Hours | Material densification & Fe3+ stabilization |
| Defect Control | Variable | Inert Gas | Continuous | Prevention of oxygen ingress & phase purity |
Elevate Your Materials Synthesis with KINTEK
Precise atmosphere control is the difference between a successful perovskite synthesis and a failed experiment. KINTEK provides industry-leading Tube, Muffle, Vacuum, and CVD systems designed to meet the most rigorous research standards.
Backed by expert R&D and advanced manufacturing, our furnaces are fully customizable to handle specific requirements like gold-foil liners or ultra-pure gas flow systems. Ensure your materials achieve perfect stoichiometry and phase stability with our high-temp solutions.
Ready to optimize your lab's thermal processing? Contact KINTEK Today to Discuss Your Custom Solution
Visual Guide
References
- Christian Berger, Rotraut Merkle. Ion transport in dry and hydrated Ba<sub>0.95</sub>La<sub>0.05</sub>(Fe<sub>1−<i>x</i></sub>Y<sub><i>x</i></sub>)O<sub>3−<i>δ</i></sub> and implications for oxygen electrode kinetics of protonic ceramic cells. DOI: 10.1039/d5ta03014e
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why is a quartz tube fixed-bed reactor ideal for VOC/Hydrogen combustion? Unlock High-Temp Precision & Stability
- How does a double tube high-temperature furnace achieve precise temperature control? Optimize Your Biochar Production
- What are the key factors to consider when choosing a vertical tube furnace? Ensure Optimal Performance for Your Lab
- What materials are used for a tube furnace heating chamber? Optimize for temperature, purity, and durability.
- What is the importance of segmented temperature control in a tube furnace for Cu/Zn-SAN? Master Atomic Dispersion
- What role does a quartz tube furnace play in the carbonization of nitrogen-doped carbon? Optimize Your Material Synthesis
- What critical reaction conditions are provided by a tube furnace for NiS2 synthesis? Achieve Pure Phase Results
- Why Use a Programmable Tube Furnace for Ni-WOx/SAPO-11 Calcination? Ensure Catalyst Purity & Performance



















