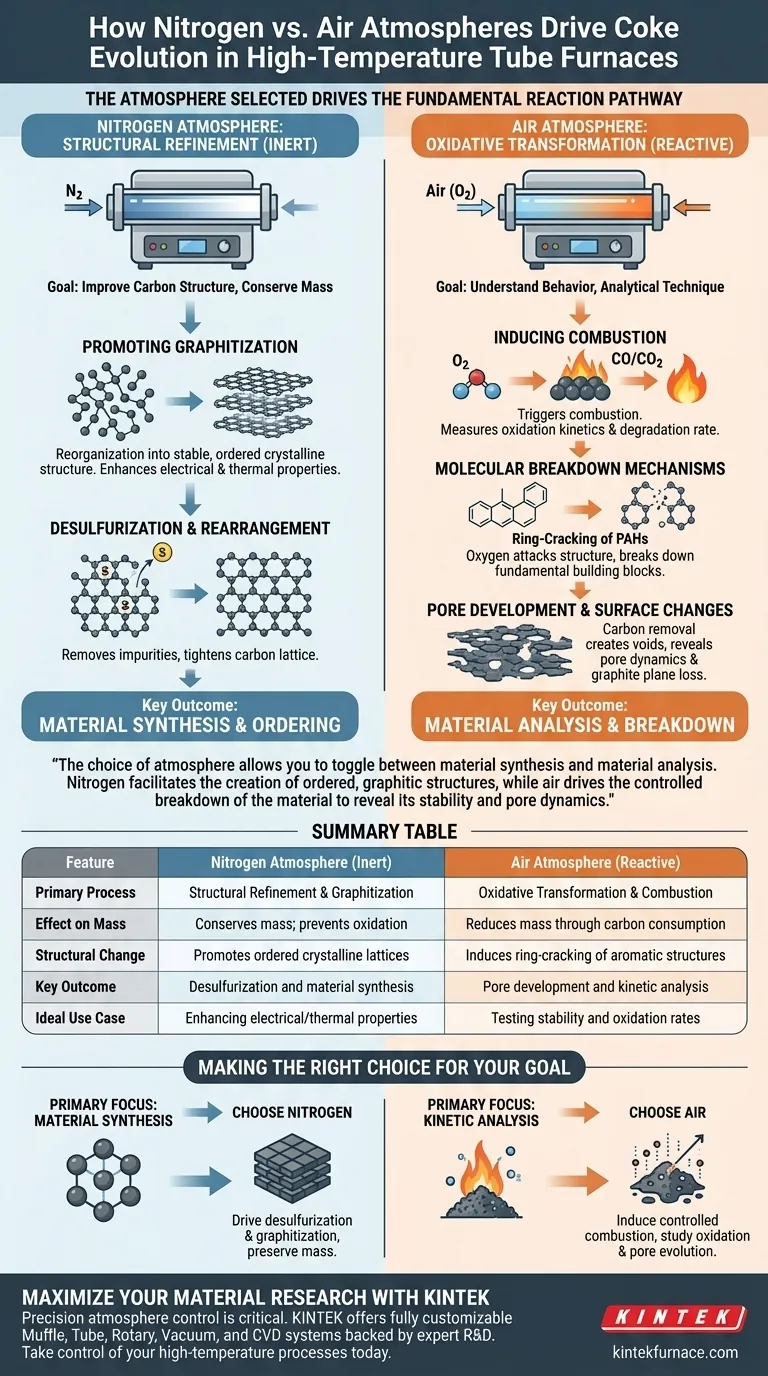
The atmosphere selected drives the fundamental reaction pathway of coke during high-temperature treatment. In an inert nitrogen atmosphere, the process focuses on conservation and ordering, leading to structural rearrangement and graphitization without chemical loss. Conversely, an air atmosphere acts as a reactive agent, introducing oxygen to induce combustion, which facilitates the study of oxidation kinetics and pore formation.
The choice of atmosphere allows you to toggle between material synthesis and material analysis. Nitrogen facilitates the creation of ordered, graphitic structures, while air drives the controlled breakdown of the material to reveal its stability and pore dynamics.

Nitrogen Atmosphere: Structural Refinement
When treating coke under nitrogen, the primary goal is usually to improve the quality of the carbon structure without reducing its mass through burning.
Promoting Graphitization
Nitrogen provides an inert environment that prevents oxidation. This allows the carbon atoms to reorganize into a more stable, ordered crystalline structure. The result is the promotion of graphitization, enhancing the material's electrical and thermal properties.
Desulfurization and Rearrangement
Beyond simple ordering, the thermal energy in a nitrogen environment drives chemical purification. The process facilitates desulfurization, removing impurities from the coke matrix. Simultaneously, structural rearrangement occurs, tightening the carbon lattice.
Air Atmosphere: Oxidative Transformation
Treating coke in air is generally an analytical technique rather than a synthesis method. It is used to understand how the material behaves when stressed by oxygen.
Inducing Combustion
The presence of oxygen in the air stream immediately triggers combustion at high temperatures. This allows researchers to measure oxidation kinetics, determining how fast the coke reacts and degrades under heat.
Molecular Breakdown mechanisms
The degradation process in air is specific and observable. Oxygen attacks the molecular structure, causing the ring-cracking of polycyclic aromatic hydrocarbons (PAHs). This breaks down the fundamental building blocks of the coke.
Pore Development and Surface Changes
As combustion proceeds, carbon is removed from the solid phase as gas (CO or CO2). This removal creates voids, allowing for the study of pore development. Additionally, researchers can observe the gradual disappearance of graphite planes as the ordered layers are stripped away by oxidation.
Understanding the Trade-offs
Selecting the wrong atmosphere will result in a completely different material outcome or data set.
Material Yield vs. Reactivity Data
Nitrogen creates a "safe" zone for the material to evolve internally. The trade-off is that it provides no information regarding reactivity or stability in harsh environments.
Air provides critical data on stability and porosity but results in the destructive consumption of the sample. You cannot use air if your goal is to harvest a high-yield carbon product at the end of the treatment.
Making the Right Choice for Your Goal
To determine which atmosphere is appropriate for your specific application, assess your desired output.
- If your primary focus is Material Synthesis: Choose nitrogen to drive desulfurization and graphitization while preserving the material's mass.
- If your primary focus is Kinetic Analysis: Choose air to induce controlled combustion, allowing you to study oxidation rates and pore evolution.
The atmosphere is not just a passive medium; it is the active switch that determines whether you are building a graphitic structure or dissecting it.
Summary Table:
| Feature | Nitrogen Atmosphere (Inert) | Air Atmosphere (Reactive) |
|---|---|---|
| Primary Process | Structural Refinement & Graphitization | Oxidative Transformation & Combustion |
| Effect on Mass | Conserves mass; prevents oxidation | Reduces mass through carbon consumption |
| Structural Change | Promotes ordered crystalline lattices | Induces ring-cracking of aromatic structures |
| Key Outcome | Desulfurization and material synthesis | Pore development and kinetic analysis |
| Ideal Use Case | Enhancing electrical/thermal properties | Testing stability and oxidation rates |
Maximize Your Material Research with KINTEK
Precision atmosphere control is critical for successful coke evolution and carbon synthesis. At KINTEK, we empower researchers with advanced thermal solutions backed by expert R&D and manufacturing.
Whether you require an inert environment for graphitization or a reactive one for oxidative analysis, our Muffle, Tube, Rotary, Vacuum, and CVD systems are fully customizable to meet your unique laboratory needs.
Take control of your high-temperature processes today. Contact our expert team to discover how KINTEK's high-performance furnaces can enhance your lab's efficiency and precision.
Visual Guide
References
- P. Nanthagopal R. Sachithananthan. Analytical Review on Impact of Catalytic Coke Formation on Reactor Surfaces During the Thermal Cracking Process. DOI: 10.5281/zenodo.17985551
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why is it necessary to use a high-temperature furnace to pre-fire porous alumina substrates for alloy wettability?
- What safety measures are critical for atmosphere furnace operation? Ensure Explosion Prevention and Operator Safety
- What are the characteristics and applications of exothermic atmospheres in furnaces? Optimize Metal Heat Treatment
- What industries benefit the most from using argon in furnaces? Ensure Material Integrity in High-Stakes Applications
- How is a protective atmosphere contained in a furnace? Engineered Seals and Positive Pressure Explained
- What components make up the atmosphere control system of the box type annealing atmosphere furnace? Discover Key Parts for Precise Heat Treatment
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality
- What is the purpose of an atmosphere furnace? Control Gas Environments for Superior Material Processing



















