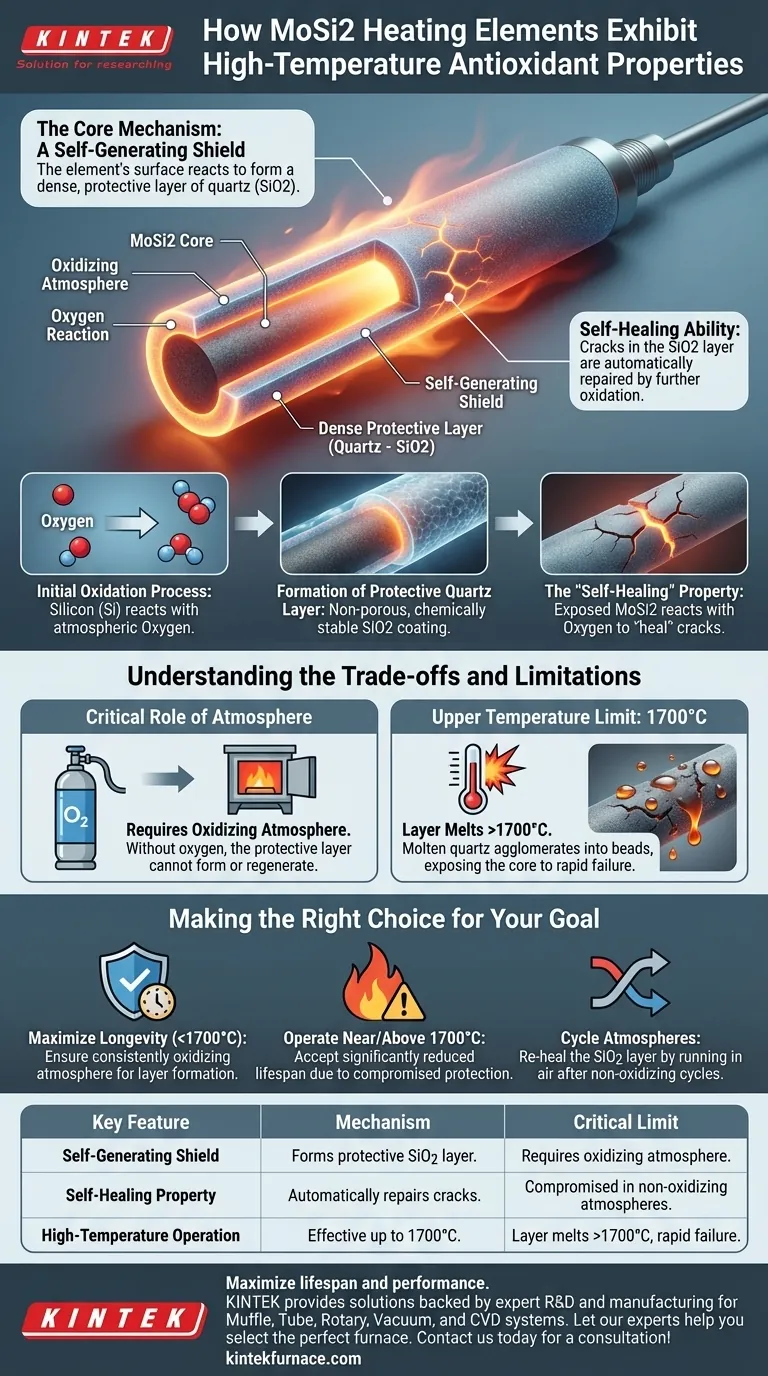
The remarkable high-temperature antioxidant property of MoSi2 heating elements is not inherent to the material itself, but rather a result of a dynamic, self-generating shield. In an oxidizing atmosphere, the element's surface reacts to form a dense, protective layer of quartz (silicon dioxide, SiO2), which acts as a physical barrier against further oxidation and degradation.
At its core, a MoSi2 element protects itself by creating its own glass-like (SiO2) coating. This self-healing ability is the key to its long life at extreme temperatures, but this same mechanism also defines its operational limits and failure points.

The Core Mechanism: A Self-Generating Shield
To understand the durability of MoSi2 elements, you must first understand the process by which they protect themselves from their own harsh operating environment.
The Initial Oxidation Process
When a new MoSi2 element is heated in the presence of oxygen, the silicon (Si) within the material readily reacts with atmospheric oxygen. This chemical reaction forms a new compound on the surface: silicon dioxide (SiO2), commonly known as quartz or silica.
Formation of the Protective Quartz Layer
This SiO2 layer is non-porous and chemically stable, forming a dense, glass-like coating over the entire hot zone of the element. It effectively seals the underlying, reactive molybdenum disilicide from any further contact with oxygen, halting the oxidation process.
The "Self-Healing" Property
The most critical feature of this process is its regenerative nature. If the protective SiO2 layer develops a crack or spalls due to thermal shock, the newly exposed MoSi2 will immediately react with oxygen to "heal" the breach, reforming the protective layer.
Understanding the Trade-offs and Limitations
This protective mechanism is incredibly effective, but it is not infallible. Its reliability is entirely dependent on specific operating conditions, and understanding these limits is crucial for preventing premature failure.
The Critical Role of Atmosphere
The formation of the SiO2 shield is entirely dependent on the presence of an oxidizing atmosphere. Without sufficient oxygen, the protective layer cannot form or regenerate, leaving the element vulnerable to degradation.
The Upper Temperature Limit
According to a fundamental principle of its operation, the protective quartz layer melts when the element's temperature exceeds 1700°C.
The Failure Mechanism Above 1700°C
Once the SiO2 melts, it no longer exists as a uniform coating. Due to surface tension, the molten quartz agglomerates into small drops or beads. This breaks the protective barrier, exposing the core element to the atmosphere and leading to rapid failure if operation at this temperature is sustained.
Making the Right Choice for Your Goal
Properly managing the environment of your MoSi2 elements is the single most important factor in maximizing their operational lifespan. Your application's specific goals will dictate your operational strategy.
- If your primary focus is maximum longevity below 1700°C: Always ensure a consistently oxidizing atmosphere to allow the protective SiO2 layer to form and regenerate as needed.
- If your process requires operating near or above 1700°C: You must accept a significantly reduced element lifespan, as the protective mechanism is compromised at these temperatures.
- If you cycle between different atmospheres: Be aware that operating in a non-oxidizing environment can degrade the SiO2 layer, and you may need to run the element in air to "re-heal" the coating before returning to high-temperature use.
By understanding that you are managing a dynamic, self-healing shield, you can directly influence the performance and durability of your heating elements.
Summary Table:
| Key Feature | Mechanism | Critical Limit |
|---|---|---|
| Self-Generating Shield | Forms a protective SiO2 layer in oxidizing atmospheres. | Requires oxygen to form and regenerate. |
| Self-Healing Property | Automatically repairs cracks in the SiO2 coating. | Compromised in non-oxidizing atmospheres. |
| High-Temperature Operation | Effective protection up to 1700°C. | Layer melts above 1700°C, leading to rapid failure. |
Maximize the lifespan and performance of your high-temperature processes.
Understanding the delicate balance of MoSi2 heating elements is key to their longevity. At KINTEK, we don't just sell furnaces; we provide solutions. Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all featuring robust heating elements and customizable designs for your unique needs.
Let our experts help you select the perfect furnace and optimize your operating conditions. Contact us today for a consultation!
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why are silicon carbide heating elements essential in high-temperature industries? Unlock Reliable, Extreme Heat Solutions
- Why are SIC heating elements resistant to chemical corrosion? Discover the Self-Protecting Mechanism
- Why is silicon carbide resistant to chemical reactions in industrial furnaces? Unlock Durable High-Temp Solutions
- What are the advantages of using high purity green silicon carbide powder in heating elements? Boost Efficiency and Lifespan
- What are the properties and applications of silicon carbide (SiC)? Unlock High-Temperature Performance



















