The exceptional chemical resistance of silicon carbide (SiC) in industrial furnaces stems from a combination of its inherent atomic structure and its ability to form a protective surface shield. At its core, the incredibly strong covalent bond between silicon and carbon atoms requires immense energy to break. This is complemented by a self-generating passive layer of silicon dioxide (SiO₂) that forms on its surface, effectively insulating it from the surrounding environment.
Silicon carbide's durability is not a single property, but a two-part defense system. Its fundamental strength comes from its stable atomic bonds, while its practical resilience in furnaces comes from a thin, self-healing layer of glass (silicon dioxide) that forms on its surface at high temperatures.

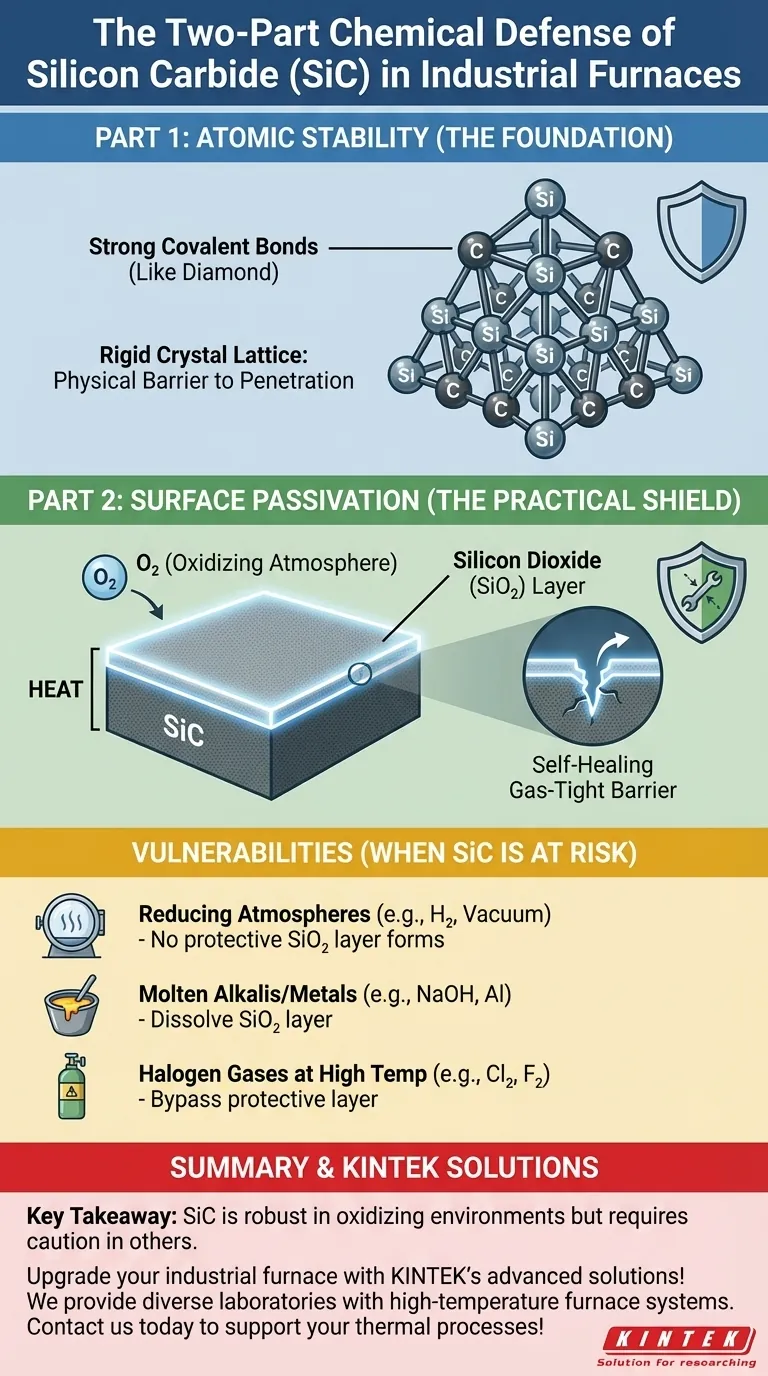
The Foundation: Atomic Stability
The root of SiC's resilience lies in its atomic configuration. Unlike metals, which are held together by a loose "sea" of electrons, SiC's atoms are locked into a rigid and powerful structure.
The Strength of the Covalent Bond
Silicon and carbon atoms share electrons in a strong covalent bond. This type of bond is one of the most stable in chemistry, similar to the bonds that give diamond its legendary hardness. Breaking this bond requires a significant amount of energy, making SiC inherently non-reactive under most conditions.
The Rigid Crystal Structure
These strong bonds arrange the atoms into a tightly packed tetrahedral crystal lattice. This rigid structure leaves very little room for foreign atoms or corrosive molecules to penetrate the material and initiate a chemical reaction. It creates a physical barrier at the atomic level.
The Practical Shield: Surface Passivation
While atomic stability is the foundation, the true key to SiC's performance in furnaces is its ability to protect itself. This process is known as passivation.
Forming the Silicon Dioxide (SiO₂) Layer
When silicon carbide is heated in an atmosphere containing oxygen (like air), the silicon at the surface reacts with the oxygen. This reaction forms a thin, dense, and highly stable layer of silicon dioxide (SiO₂), which is essentially a form of quartz or glass.
How the Protective Layer Works
This SiO₂ layer is non-porous and adheres strongly to the SiC substrate. It acts as a gas-tight barrier, physically separating the underlying silicon carbide from the reactive gases in the furnace. If the layer is ever scratched or damaged at high temperatures, the exposed SiC will simply react with more oxygen to "heal" the shield, making it a remarkably effective and renewable defense.
Understanding the Trade-offs: When SiC is Vulnerable
No material is perfect, and understanding SiC's limitations is critical for proper application. Its chemical resistance is highly dependent on the furnace environment.
The Role of Atmosphere
The protective SiO₂ layer only forms in an oxidizing atmosphere. In a reducing atmosphere (like pure hydrogen or a hard vacuum), this layer cannot form or can be stripped away. This leaves the SiC more vulnerable to reacting with other materials.
Attack from Molten Alkalis and Metals
The SiO₂ layer, while resistant to acids, can be dissolved by strong molten alkalis (like sodium hydroxide) and certain molten metals (like aluminum). Once this protective layer is gone, these aggressive chemicals can directly attack the silicon carbide itself.
Halogen Gases at High Temperatures
At very high temperatures, halogen gases like chlorine and fluorine are reactive enough to bypass the protective layer and attack the SiC, forming volatile silicon halides. This is a specific failure mode to consider in chemical processing applications.
Applying This to Your Furnace Environment
Your choice of material must be aligned with the chemical conditions of your process. Understanding SiC's defense mechanism allows you to predict its performance and ensure process integrity.
- If your primary focus is high-temperature operation in air or an oxidizing atmosphere: SiC is an outstanding choice, as the environment continuously reinforces the protective SiO₂ layer that ensures its longevity.
- If your primary focus is working with molten alkalis (caustics) or reactive metals: SiC is likely a poor choice, as these materials will strip its protective layer and cause rapid degradation.
- If your primary focus is operating in a hard vacuum or reducing atmosphere: SiC remains structurally stable, but you lose the benefit of the self-healing oxide shield, which must be factored into lifetime and contamination calculations.
By understanding both the innate strength and the environmental dependencies of silicon carbide, you can confidently engineer a more reliable and effective thermal process.
Summary Table:
| Factor | Role in Chemical Resistance |
|---|---|
| Atomic Structure | Strong covalent bonds and rigid crystal lattice resist penetration and reactions |
| Surface Passivation | Forms a protective SiO₂ layer in oxidizing atmospheres, self-healing if damaged |
| Environmental Dependencies | Vulnerable in reducing atmospheres, molten alkalis, and halogen gases at high temperatures |
Upgrade your industrial furnace with KINTEK's advanced solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with high-temperature furnace systems like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our deep customization capability ensures precise fit for your unique experimental needs, enhancing durability and efficiency. Contact us today to discuss how we can support your thermal processes!
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What are the advantages of using high purity green silicon carbide powder in heating elements? Boost Efficiency and Lifespan
- What makes silicon carbide heating elements resistant to chemical corrosion? Discover the Protective Oxide Layer
- Why are silicon carbide heating elements essential in high-temperature industries? Unlock Reliable, Extreme Heat Solutions
- Why are SiC heating elements considered environmentally friendly? Discover Their Eco-Efficiency & Lifespan Insights
- Why are SIC heating elements resistant to chemical corrosion? Discover the Self-Protecting Mechanism



















