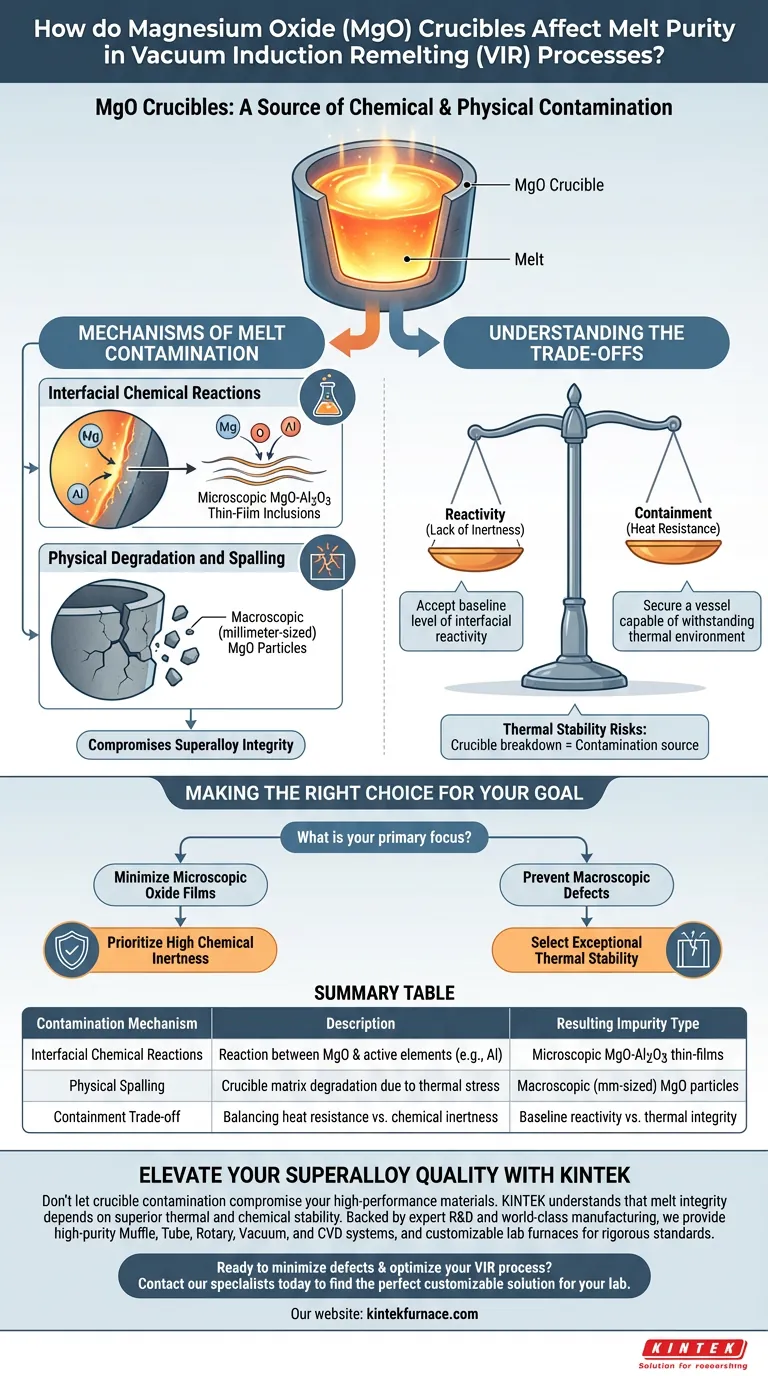
Magnesium oxide (MgO) crucibles directly influence melt purity by serving as a source of both chemical and physical contamination during Vacuum Induction Remelting (VIR). While acting as the primary containment vessel, the crucible is not passive; it interacts with the melt to introduce oxide films through chemical reactions and larger inclusions through physical degradation.
The integrity of your superalloy is heavily dependent on the crucible-melt interface, where MgO crucibles can introduce impurities through thin-film chemical reactions and physical spalling.
Mechanisms of Melt Contamination
The purity of an alloy processed in VIR is compromised by two distinct mechanisms related to the MgO crucible. Understanding the difference between chemical and physical contamination is vital for quality control.
Interfacial Chemical Reactions
At high processing temperatures, the inner surface of the MgO crucible is chemically active. It can undergo interfacial reactions with highly active alloying elements present in the melt.
These reactions often result in the formation of thin-film inclusions, specifically magnesium oxide-aluminum oxide (MgO-Al2O3) compounds. These microscopic impurities are generated directly at the boundary where the melt meets the containment wall.
Physical Degradation and Spalling
Beyond chemical reactions, the physical structure of the crucible matrix can degrade during the process. This phenomenon is known as localized spalling.
When spalling occurs, millimeter-sized magnesium oxide inclusions are released from the crucible wall directly into the alloy. Unlike thin films, these are macroscopic particles that can significantly impair the material properties of the final product.
Understanding the Trade-offs
Selecting a crucible for VIR involves balancing the need for containment with the risk of contamination.
Reactivity vs. Containment
While MgO provides the necessary heat resistance for induction remelting, its lack of total chemical inertness is a significant liability. The trade-off lies in accepting a baseline level of interfacial reactivity to secure a vessel capable of withstanding the thermal environment.
Thermal Stability Risks
The risk of spalling highlights a critical trade-off regarding thermal stability. A crucible matrix that cannot withstand the thermal stresses of the process will physically break down, turning the containment vessel itself into a contaminant.
Making the Right Choice for Your Goal
To mitigate these risks, your selection of crucible materials must be driven by the specific quality requirements of your alloy.
- If your primary focus is minimizing microscopic oxide films: Prioritize crucible materials with high chemical inertness to reduce interfacial reactions with active alloying elements.
- If your primary focus is preventing macroscopic defects: Select crucible matrices with exceptional thermal stability to prevent localized spalling and the release of large MgO particles.
The quality of your final superalloy is ultimately defined by the chemical and physical stability of the crucible containing it.
Summary Table:
| Contamination Mechanism | Description | Resulting Impurity Type |
|---|---|---|
| Interfacial Chemical Reactions | Reaction between MgO and active alloying elements (e.g., Al) | Microscopic MgO-Al2O3 thin-film inclusions |
| Physical Spalling | Degradation of the crucible matrix due to thermal stress | Macroscopic (millimeter-sized) MgO particles |
| Containment Trade-off | Balancing heat resistance vs. chemical inertness | Baseline reactivity vs. thermal vessel integrity |
Elevate Your Superalloy Quality with KINTEK
Don't let crucible contamination compromise your high-performance materials. At KINTEK, we understand that the integrity of your melt depends on superior thermal and chemical stability. Backed by expert R&D and world-class manufacturing, we provide high-purity Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable lab high-temperature furnaces designed to meet your most rigorous purity standards.
Ready to minimize defects and optimize your VIR process?
Contact our specialists today to find the perfect customizable solution for your lab.
Visual Guide
References
- Solidification and Casting of Metals and Alloys. DOI: 10.3390/met15010087
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What are the benefits of induction furnace? Achieve Unmatched Efficiency & Purity in Metal Melting
- What role does a vacuum arc melting furnace with a non-consumable electrode play? Key to CuAlMn Shape Memory Alloys
- What factors should be considered when selecting an induction melting furnace? A Guide to Maximizing ROI
- Why is an argon atmosphere maintained during VIM of Chromium-Silicon alloys? Prevent High Chromium Loss
- What other metal alloys benefit from vacuum induction melting? Unlock Purity for Reactive Metals and Specialty Alloys
- What are the advantages of using an induction-heated vacuum furnace? Achieve Purity and Precision in Material Processing
- What is the working principle of an induction furnace for melting gold? Discover Fast, Clean Melting for Precious Metals
- What are the main differences between VIM and Arc Melting furnaces? Choose the Right Melting Tech for Your Alloys



















