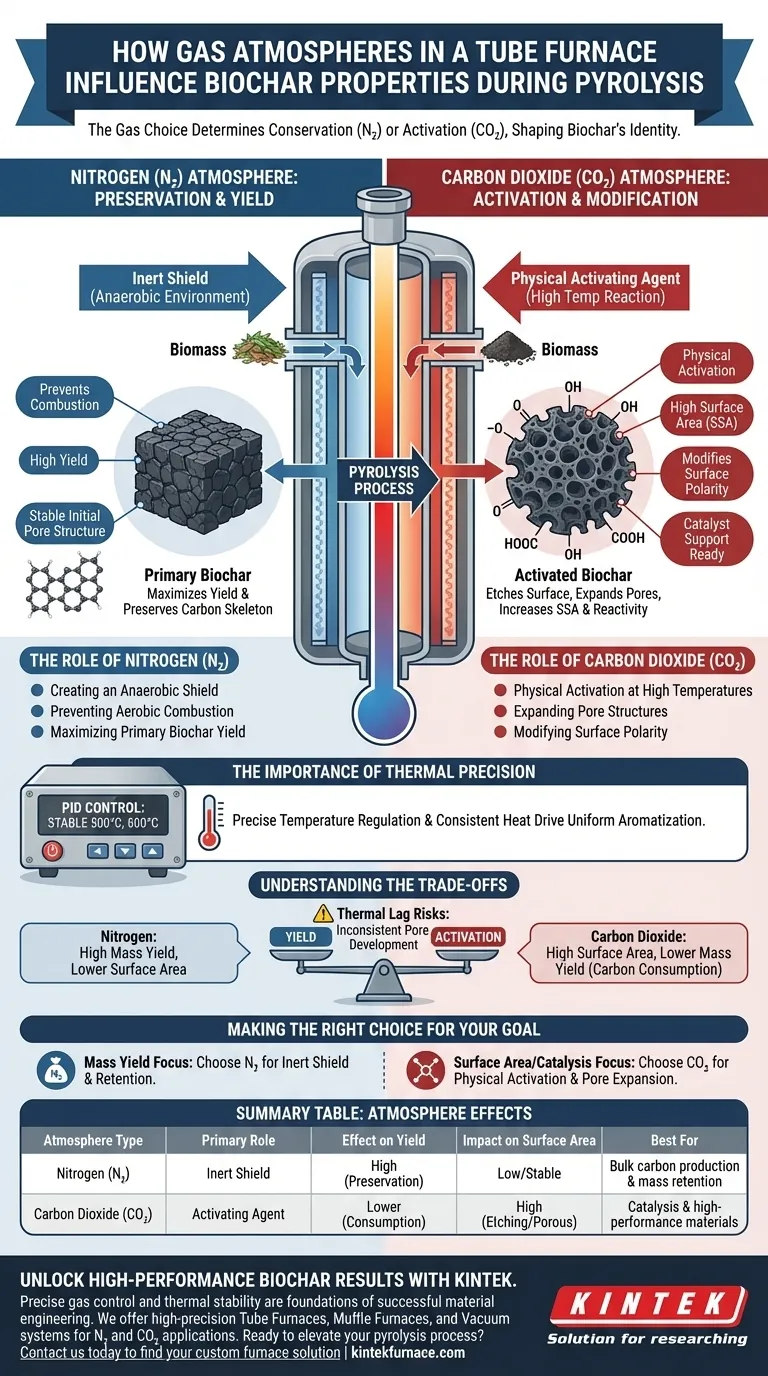
The gas atmosphere selected for a tube furnace determines the fundamental chemical and physical identity of the resulting biochar. Specifically, the choice between nitrogen and carbon dioxide dictates whether the process focuses on conservation or activation. Nitrogen creates a protective, inert environment that maximizes yield, while carbon dioxide actively modifies the carbon structure to significantly increase surface area and chemical reactivity.
By manipulating the gas atmosphere, you shift the pyrolysis process from simple carbonization to advanced material engineering. Nitrogen preserves the carbon skeleton for high yields, whereas carbon dioxide etches the surface to create the porosity and functional groups required for high-performance applications like catalysis.
The Role of Nitrogen ($N_2$): Preservation and Yield
Creating an Anaerobic Shield
High-purity nitrogen functions primarily as an inert protective gas. Its presence ensures a strictly anaerobic environment within the tube furnace.
Preventing Aerobic Combustion
By displacing oxygen, nitrogen prevents the biomass from undergoing aerobic combustion during heating. This is critical for ensuring the biomass is fully carbonized rather than burned to ash.
Maximizing Primary Biochar Yield
Because nitrogen does not chemically react with the biomass at standard pyrolysis temperatures, it preserves the carbon mass. This results in a high yield of "primary biochar" with a stable, initial pore structure.
The Role of Carbon Dioxide ($CO_2$): Activation and Modification
Physical Activation at High Temperatures
Unlike nitrogen, carbon dioxide acts as a physical activating agent when introduced at high temperatures. It enters the furnace not to protect the material, but to transform it.
Expanding Pore Structures
$CO_2$ reacts with the biochar surface, effectively "etching" the carbon. This reaction expands the pore structure, leading to a dramatic increase in Specific Surface Area (SSA).
Modifying Surface Polarity
The interaction between $CO_2$ and the carbon matrix facilitates the formation of oxygen-containing functional groups. This alters the biochar’s polarity, making it more chemically active and suitable for use as a catalyst support.
The Importance of Thermal Precision
Precise Temperature Regulation
The gas atmosphere relies on the furnace's ability to maintain precise thermal conditions. Advanced tube furnaces utilize PID control systems to lock in specific temperatures (e.g., 500°C or 600°C).
Impact on Aromatization
This stable thermal environment, combined with the chosen gas, drives the degree of aromatization. Consistent heat ensures that surface chemical properties develop uniformly across the batch.
Understanding the Trade-offs
Yield vs. Surface Area
There is an inherent trade-off between yield and activation. Nitrogen ensures the highest mass yield but results in biochar with lower surface area and lower reactivity.
Activation Consumption
Conversely, using Carbon Dioxide to increase surface area comes at the cost of carbon mass. The activation process physically consumes parts of the carbon structure to create pores, resulting in a lower overall yield.
Thermal Lag Risks
While high-performance insulation allows for rapid heating rates (approx. 60°C/min), precise control is vital. If the temperature fluctuates, the interaction between the gas and the biochar becomes unpredictable, leading to inconsistent pore development.
Making the Right Choice for Your Goal
To select the correct atmosphere, you must define the intended application of your biochar.
- If your primary focus is Mass Yield: Choose a Nitrogen ($N_2$) atmosphere to create an inert shield that maximizes carbon retention and structural stability.
- If your primary focus is Surface Area (SSA) or Catalysis: Choose a Carbon Dioxide ($CO_2$) atmosphere to physically activate the material, expand pore structures, and increase oxygen functional groups.
The gas atmosphere is not merely a background condition; it is an active tool that dictates whether you are manufacturing a bulk carbon product or a high-performance chemical material.
Summary Table:
| Atmosphere Type | Primary Role | Effect on Yield | Impact on Surface Area | Best For |
|---|---|---|---|---|
| Nitrogen (N2) | Inert Shield | High (Preservation) | Low/Stable | Bulk carbon production & mass retention |
| Carbon Dioxide (CO2) | Activating Agent | Lower (Consumption) | High (Etching/Porous) | Catalysis & high-performance materials |
Unlock High-Performance Biochar Results with KINTEK
Precise gas control and thermal stability are the foundations of successful material engineering. At KINTEK, we understand that your research demands exacting standards. Backed by expert R&D and manufacturing, we offer high-precision Tube Furnaces, Muffle Furnaces, and Vacuum systems designed to handle complex gas atmospheres like $N_2$ and $CO_2$ with ease.
Whether you need to maximize your carbon yield or engineer advanced porous structures for catalysis, our customizable lab high-temp furnaces provide the PID-controlled accuracy you need to succeed.
Ready to elevate your pyrolysis process? Contact us today to find your custom furnace solution!
Visual Guide
References
- Huiying Zhang, Weifeng Chen. Roles of biochars’ properties in their water-holding capacity and bound water evaporation: quantitative importance and controlling mechanism. DOI: 10.1007/s42773-024-00317-2
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What materials besides metals benefit from inert atmosphere heat treating? Protect High-Performance Polymers Like PTFE
- How does a reactive rapid thermal annealing furnace contribute to phosphosulfide crystallization? Expert Insights
- What factors should be considered when selecting a controlled atmosphere furnace? Ensure Process Success with Expert Guidance
- Why is it necessary to use an atmosphere furnace with argon gas? Ensure Precise Alloy Austenitization & Protection
- What is the purpose of a chemically reactive atmosphere in material processing? Achieve Precise Surface Modification for Enhanced Performance
- What is an atmosphere box furnace and what are its primary uses? Essential for Controlled Heat Processing
- Why is a gas nitriding furnace equipped with an atmosphere control system used for titanium alloys? Ensure Precision
- What are the key components of an inert atmosphere furnace? Essential Parts for Contamination-Free Heating



















