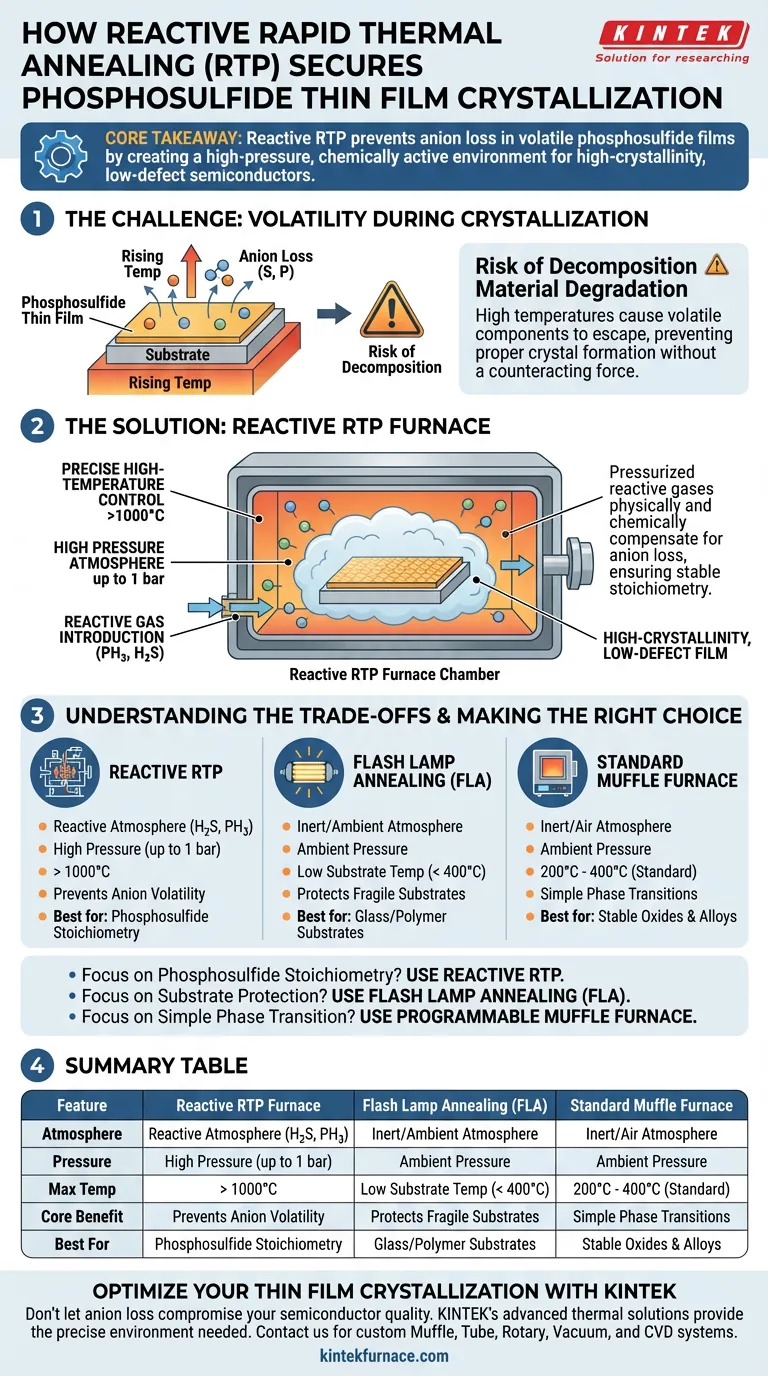
A reactive rapid thermal annealing (RTP) furnace secures the crystallization process by maintaining a high-pressure, chemically active environment that prevents the film from degrading at high temperatures. It combines precise thermal control exceeding 1000°C with the introduction of specific reactive gases to ensure the material retains its intended chemical composition.
Core Takeaway The primary challenge in crystallizing phosphosulfide films is the volatility of their components. Reactive RTP solves this by pressurizing the chamber with reactive gases, physically and chemically compensating for anion loss to produce high-crystallinity, low-defect semiconductors.

The Challenge: Volatility During Crystallization
The Risk of Anion Loss
Phosphosulfide thin films contain volatile components that become unstable when heated.
As temperatures rise to the levels required for crystallization, these films naturally tend to lose anions (such as sulfur or phosphorus).
Preventing Decomposition
Without a counteracting force, this loss leads to material decomposition rather than proper crystal formation.
Standard annealing methods, which often rely on inert atmospheres or vacuums, are insufficient to stop this chemical breakdown in phosphosulfides.
How Reactive RTP Solves the Problem
Creating a Reactive Atmosphere
The furnace enables the introduction of reactive gases, specifically phosphine (PH3) or hydrogen sulfide (H2S).
This creates a chemical environment that actively supplies the necessary anions during the heating process.
Utilizing High Pressure
The system operates under a controlled high-pressure atmosphere up to 1 bar.
This pressure works in tandem with the reactive gases to effectively compensate for the loss of volatile anions, forcing the chemistry to remain stable.
Precise High-Temperature Control
The furnace facilitates precise temperature cycles capable of exceeding 1000°C.
This high thermal energy is necessary to drive the structural arrangement of the film into a state of high crystallinity.
Understanding the Trade-offs
Substrate Limitations
While Reactive RTP is powerful, the high temperatures (>1000°C) required for phosphosulfides can be damaging to certain substrates.
In contrast, techniques like Flash Lamp Annealing (FLA) are better suited for low-melting-point substrates (like glass) because they keep the substrate below 400°C, though they may lack the reactive atmosphere control.
Complexity vs. Simplicity
Reactive RTP involves handling toxic, high-pressure gases (PH3, H2S) to manage stoichiometry.
Simpler methods, such as muffle furnaces or laboratory tube furnaces, operate at lower temperatures (200°C–400°C) in inert (Argon) or air atmospheres, which is sufficient for stable oxides or simple alloys but inadequate for volatile phosphosulfides.
Making the Right Choice for Your Goal
To select the correct annealing method, you must evaluate the volatility of your film and the thermal limits of your substrate.
- If your primary focus is Phosphosulfide Stoichiometry: Use Reactive RTP to prevent decomposition and defect formation via anion compensation.
- If your primary focus is Substrate Protection: Consider Flash Lamp Annealing (FLA) to achieve surface crystallization without deforming heat-sensitive substrates.
- If your primary focus is Simple Phase Transition: Use a Programmable Muffle Furnace for stable materials requiring lower temperatures (200°C–300°C) to minimize thermal stress.
Success in phosphosulfide fabrication depends not just on heating the film, but on chemically preserving it while it crystallizes.
Summary Table:
| Feature | Reactive RTP Furnace | Flash Lamp Annealing (FLA) | Standard Muffle Furnace |
|---|---|---|---|
| Atmosphere | Reactive (H2S, PH3) | Inert or Ambient | Inert or Air |
| Pressure | High Pressure (up to 1 bar) | Ambient | Ambient |
| Max Temp | > 1000°C | Low Substrate Temp (< 400°C) | 200°C - 400°C (Standard) |
| Core Benefit | Prevents Anion Volatility | Protects Fragile Substrates | Simple Phase Transitions |
| Best For | Phosphosulfide Stoichiometry | Glass/Polymer Substrates | Stable Oxides & Alloys |
Optimize Your Thin Film Crystallization with KINTEK
Don't let anion loss compromise your semiconductor quality. KINTEK’s advanced thermal solutions provide the precise environment needed for complex material synthesis. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique research or production requirements.
Whether you are working with volatile phosphosulfides or sensitive substrates, our engineering team is ready to help you design the perfect thermal profile. Contact KINTEK today to discuss your custom furnace needs and achieve superior material performance.
Visual Guide
References
- Lena Angelika Mittmann, Andrea Crovetto. Phosphosulfide semiconductors for optoelectronics and solar energy conversion. DOI: 10.1088/2515-7639/ad3aa3
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
People Also Ask
- What is the primary function of a controlled atmosphere device in powder metallurgy? Ensure Pure Sintering Results
- What are the five key components of atmosphere furnaces? Master Controlled Heat Treatment for Superior Results
- What types of environments can the protective atmosphere box furnace be used in? Essential for Oxidation-Free High-Temp Processes
- What materials are used for the furnace structure of the box type annealing atmosphere furnace? Discover Durable, High-Temp Solutions
- How is the box type annealing atmosphere furnace utilized in metal material research? Unlock Precision Heat Treatment
- In which fields is the inert atmosphere principle commonly applied? Discover Key Uses in Heat Treatment, Food, and More
- How does the design of a convector plate affect the thermal efficiency? Maximize Bell-Type Annealing Performance
- For what purpose is a chemically reactive atmosphere used in a furnace? To Transform Material Surfaces



















