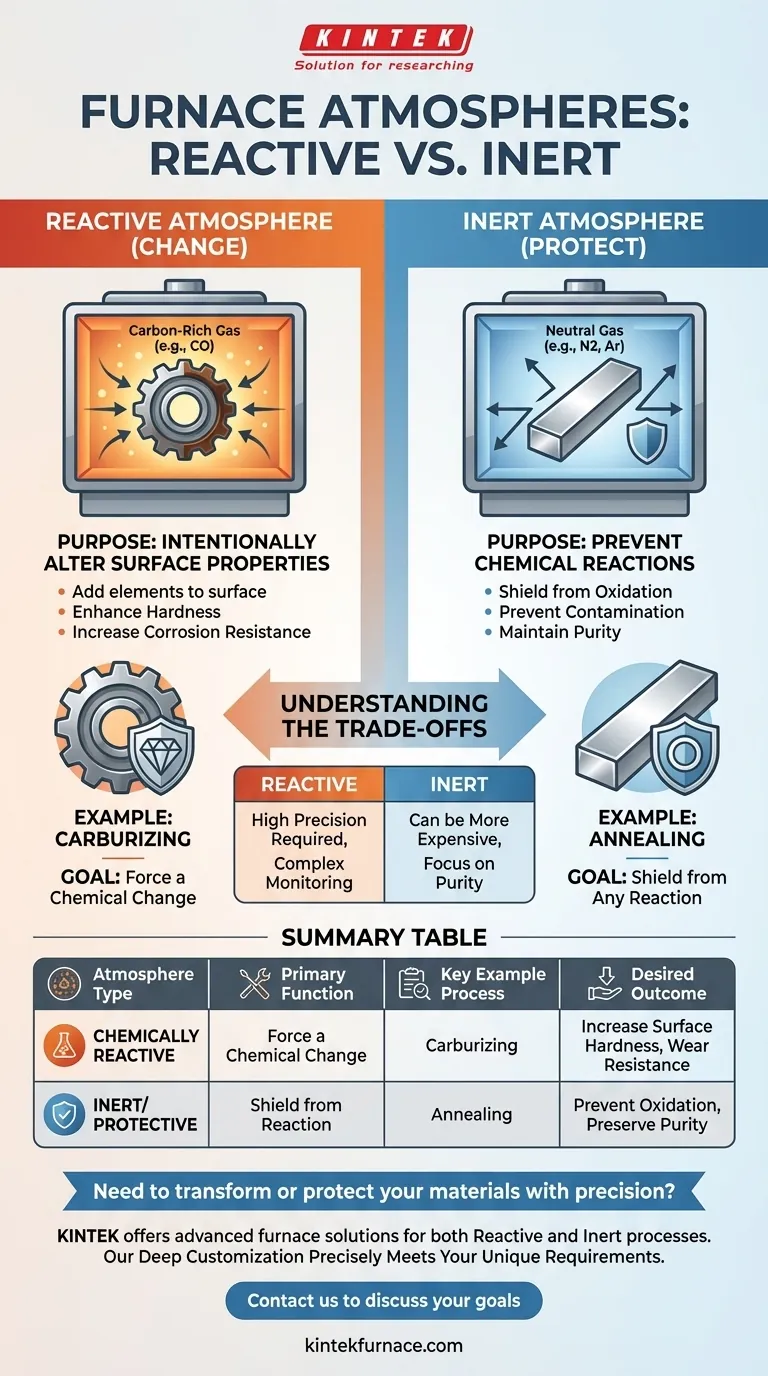
In short, a chemically reactive atmosphere is used in a furnace to intentionally cause a chemical reaction on the surface of a material. This process is a form of surface engineering, deliberately designed to add or remove elements to change the material's fundamental properties, such as increasing its surface hardness or corrosion resistance.
The core purpose of a furnace atmosphere is not always to protect. It is a critical engineering choice: you either use an atmosphere to shield the material from change (an inert atmosphere) or to force a specific change upon it (a reactive atmosphere).

The Two Functions of a Furnace Atmosphere
At high temperatures, most materials are highly susceptible to reactions with the surrounding air, particularly with oxygen. A controlled furnace atmosphere is introduced to manage these reactions, serving one of two distinct purposes.
Purpose 1: To Change the Material (Reactive Atmosphere)
A chemically reactive atmosphere is used when the goal is to fundamentally alter the surface of the processed material.
Gases are intentionally introduced to react with the workpiece, creating a new surface composition with enhanced properties. This is a common technique in metallurgy and material science.
A primary example is carburizing. In this process, a carbon-rich atmosphere (using gases like carbon monoxide) is used to diffuse carbon atoms into the surface of steel, significantly increasing its hardness and wear resistance.
Purpose 2: To Protect the Material (Inert Atmosphere)
An inert or protective atmosphere is used for the opposite reason: to prevent any chemical reactions from occurring.
Gases like nitrogen or argon are used to displace oxygen and other reactive elements. This creates a neutral environment that shields the material from oxidation, contamination, and other unwanted changes.
This approach is critical for processes like annealing, where the goal is to soften a metal and relieve internal stresses without altering its chemical makeup or surface finish.
Understanding the Trade-offs and Control
The choice of atmosphere is dictated entirely by the desired outcome, but it comes with critical considerations that demand precision.
The Challenge of Precision
Reactive atmospheres require extremely precise control. Small fluctuations in gas composition, temperature, or process time can lead to incorrect surface properties, rendering the component unusable.
Cost and Complexity
Protective atmospheres, especially those using high-purity argon, can be more expensive than reactive gas mixtures. However, reactive processes often require more sophisticated monitoring equipment and safety protocols to manage the chemical reactions safely.
Process-Specific Requirements
There is no single "best" atmosphere. The choice is fundamentally tied to the material being processed and the engineering goal. Using a reactive atmosphere when protection is needed will ruin the part, and vice-versa.
Making the Right Choice for Your Goal
Your process requirements will dictate the correct atmospheric strategy.
- If your primary focus is surface enhancement: A reactive atmosphere is the tool used to deliberately alter the material's surface chemistry, such as adding carbon for hardness.
- If your primary focus is material preservation: An inert atmosphere is the shield used to prevent oxidation and contamination during heat treatment, maintaining the material's purity.
Ultimately, the furnace atmosphere is a powerful tool, used either as a shield to protect or as a catalyst to transform.
Summary Table:
| Atmosphere Type | Primary Function | Key Example Process | Desired Outcome |
|---|---|---|---|
| Chemically Reactive | To force a chemical change on the material surface | Carburizing | Increase surface hardness, wear resistance |
| Inert/Protective | To shield the material from any chemical reaction | Annealing | Prevent oxidation, preserve material purity |
Need to transform or protect your materials with precision?
Leveraging exceptional R&D and in-house manufacturing, KINTEK provides diverse laboratories with advanced high-temperature furnace solutions. Whether your process requires a reactive atmosphere for surface engineering (like carburizing) or an inert atmosphere for material preservation (like annealing), our product line—including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems—is complemented by our strong deep customization capability to precisely meet your unique experimental requirements.
Contact us today to discuss how our furnace solutions can achieve your specific material processing goals.
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How do argon and nitrogen protect samples in vacuum furnaces? Optimize Your Thermal Process with the Right Gas
- How does the pressure range change under vacuum conditions in an atmosphere box furnace? Explore Key Shifts for Material Processing
- What are the key features of an atmosphere box furnace? Unlock Precise Heat Processing in Controlled Environments
- What are some specific applications of atmosphere furnaces in the ceramics industry? Enhance Purity and Performance
- What are the development prospects of atmosphere box furnaces in the aerospace industry? Unlock Advanced Material Processing for Aerospace Innovation



















