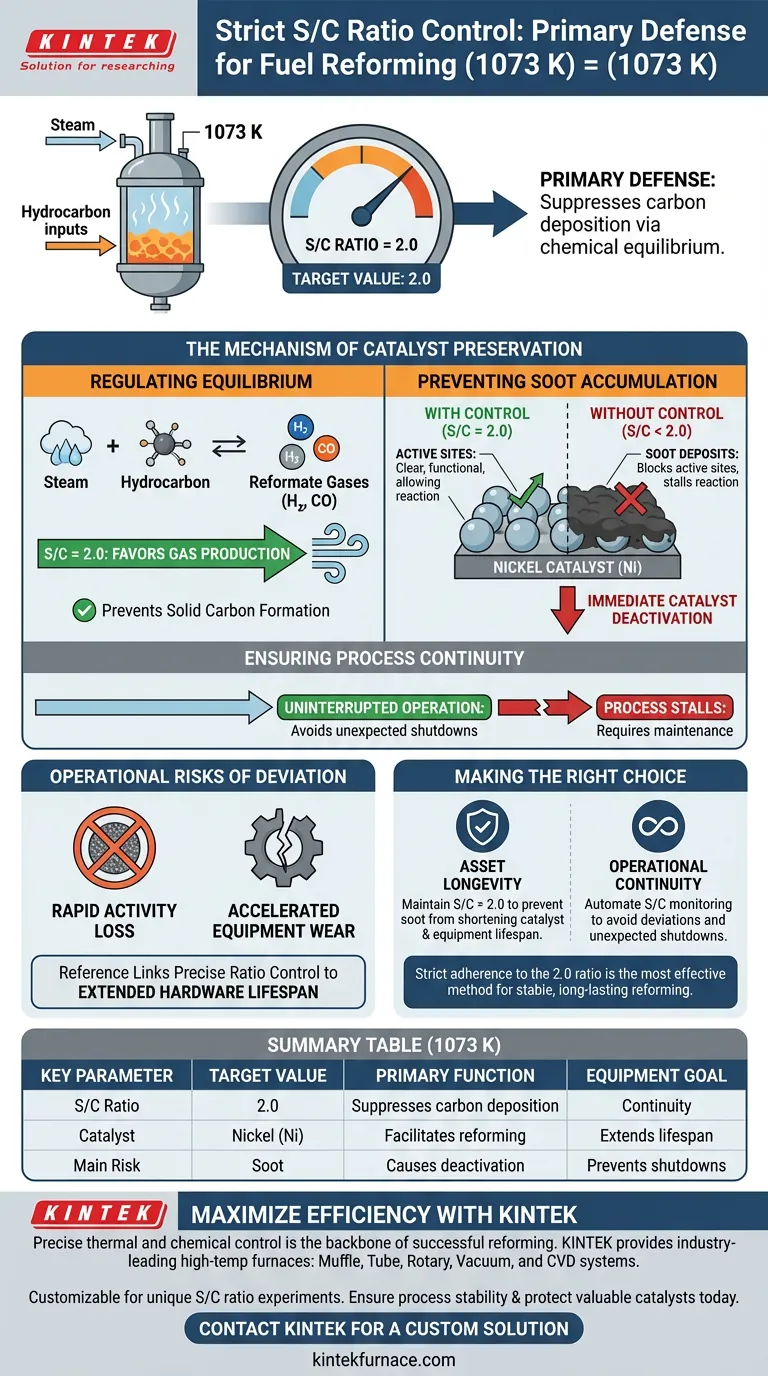
Strict control of the steam-to-carbon (S/C) ratio is the primary defense against catalyst failure during fuel reforming. At an operating temperature of 1073 K, maintaining a specific ratio of 2.0 is required to suppress carbon deposition via chemical equilibrium, effectively preventing soot from destroying the active nickel catalysts.
The primary function of the S/C ratio is not just reaction efficiency, but catalyst preservation. By preventing soot accumulation on nickel surfaces, precise ratio control ensures process continuity and maximizes the lifespan of expensive reforming equipment.

The Mechanism of Catalyst Preservation
Regulating Chemical Equilibrium
The reforming process relies on a delicate chemical balance. At 1073 K, the S/C ratio acts as a lever to manipulate this equilibrium.
By holding the ratio at 2.0, the system is chemically forced to suppress the formation of solid carbon. This specific proportion ensures that the thermodynamics of the reaction favor the production of reformate gases rather than solid byproducts.
Preventing Soot Accumulation
Without strict control, carbon precipitates out of the gas phase as soot.
This soot physically deposits onto the surface of the nickel catalysts used in the reformer. This accumulation blocks the active sites of the catalyst, rendering them unable to facilitate the reaction.
Ensuring Process Continuity
Carbon deposition is not a reversible minor nuisance; it leads to rapid catalyst deactivation.
Once the nickel is covered in soot, the reforming reaction stalls. Therefore, maintaining the ratio is a necessary condition for ensuring the continuous operation of the reformer without unexpected shutdowns.
The Operational Risks of Ratio Deviation
Immediate Catalyst Deactivation
The most significant risk in this process is the rapid loss of catalytic activity.
If the S/C ratio drops below the critical threshold of 2.0, the suppression of carbon deposition fails. This leads to immediate soot buildup, causing irreversible damage to the catalyst's efficiency.
Impact on Equipment Lifespan
The implications of the S/C ratio extend beyond the chemistry of the reaction to the physical hardware.
The reference explicitly links precise ratio control to extending the lifespan of key process equipment. Failing to control this parameter accelerates wear and necessitates premature replacement of reformer components.
Making the Right Choice for Your Goal
To ensure the stability of your fuel reforming process at 1073 K, you must prioritize the integrity of the catalyst above all else.
- If your primary focus is Asset Longevity: Maintain a strict S/C ratio of 2.0 to prevent soot from shortening the lifespan of your nickel catalysts and process equipment.
- If your primary focus is Operational Continuity: Automate the monitoring of the S/C ratio to ensure it never deviates from equilibrium requirements, thereby avoiding unexpected shutdowns due to deactivation.
Strict adherence to the 2.0 ratio is the single most effective method for guaranteeing a stable, long-lasting reforming operation.
Summary Table:
| Key Parameter | Target Value (1073 K) | Primary Function |
|---|---|---|
| S/C Ratio | 2.0 | Suppresses carbon deposition via equilibrium |
| Catalyst Material | Nickel (Ni) | Facilitates the reforming reaction |
| Main Risk | Soot Accumulation | Causes immediate catalyst deactivation |
| Equipment Goal | Continuity | Extends hardware lifespan & prevents shutdowns |
Maximize Your Reforming Efficiency with KINTEK
Precise thermal and chemical control is the backbone of successful fuel reforming. KINTEK provides industry-leading laboratory high-temp furnaces—including Muffle, Tube, Rotary, Vacuum, and CVD systems—engineered to maintain the rigorous environments your research demands. Backed by expert R&D and manufacturing, our systems are fully customizable to your unique S/C ratio experiments and catalyst testing needs.
Ensure process stability and protect your valuable catalysts today.
Contact KINTEK for a Custom Solution
Visual Guide
References
- Ivan Beloev, Iliya Iliev. Utilization of Hydrogen-Containing Gas Waste from Deep Oil Refining at a Hybrid Power Plant with a Solid Oxide Fuel Cell. DOI: 10.3390/engproc2024060005
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Kiln for Pyrolysis Plant Heating
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What is the significance of calculating AC impedance in the power control of indirect heating resistance furnaces?
- How does industrial-scale forging equipment influence the morphology of primary carbonitrides in H13 tool steel?
- What is the main benefit of using a benchtop industrial oven? Save Space and Boost Efficiency in Your Lab
- What role does an industrial fast firing furnace play in the metallization of PERT solar cells? Boost Cell Efficiency
- Why is a heating furnace set to 155 °C for sulfur melt-diffusion? Unlock Optimal Battery Material Synthesis
- Why is the high-precision control of argon (Ar) and nitrogen (N2) flow ratios critical in CrSiN-Y coating fabrication?
- Why is argon particularly attractive for industrial applications? Unlock Cost-Effective Purity and Stability
- What is the function of a laboratory drying oven in processing NdFeB waste? Ensure Purity in Rare Earth Recovery













