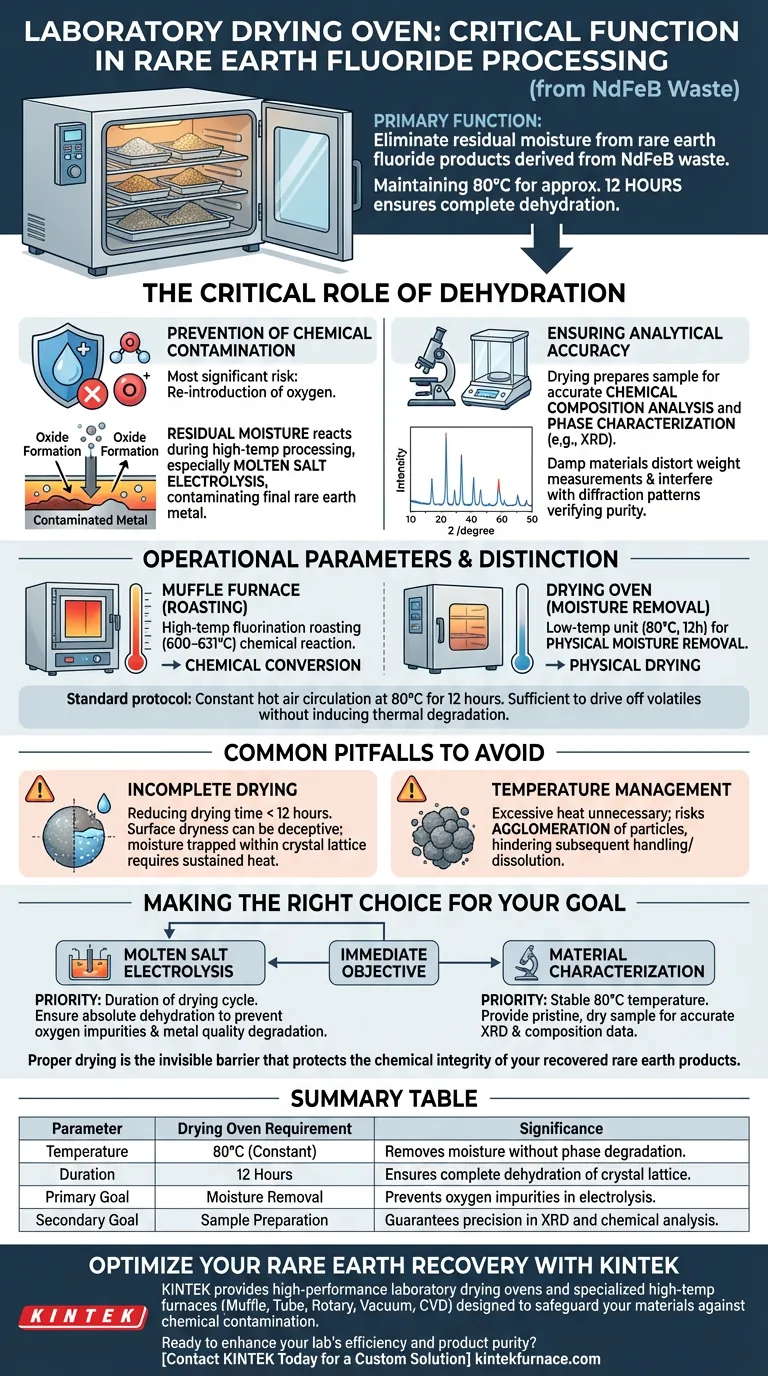
The primary function of a laboratory drying oven in this context is to eliminate residual moisture from rare earth fluoride products derived from NdFeB waste. By maintaining a stable thermal environment, typically at 80°C for approximately 12 hours, the oven ensures the material is completely dehydrated, which is a prerequisite for stabilizing the product before downstream applications.
The drying process is not merely about physical handling; it is a critical chemical safeguard. Thorough dehydration is essential to prevent the formation of oxygen impurities that can compromise subsequent molten salt electrolysis and to guarantee the accuracy of analytical characterization.

The Critical Role of Dehydration
Prevention of Chemical Contamination
The most significant risk in processing rare earth fluorides is the re-introduction of oxygen.
If residual moisture remains in the product, it can react during storage or high-temperature processing stages.
This is particularly detrimental during molten salt electrolysis, where moisture can lead to the formation of oxides, contaminating the final rare earth metal.
Ensuring Analytical Accuracy
Precise characterization is impossible with damp materials.
The drying oven prepares the sample for accurate chemical composition analysis and phase characterization techniques, such as X-ray diffraction (XRD).
Moisture in the sample would distort weight measurements and interfere with the diffraction patterns required to verify the purity of the fluoride phase.
Operational Parameters and Distinction
The Thermal Profile
The standard protocol for this specific application involves constant hot air circulation at 80°C for 12 hours.
This temperature is sufficient to drive off volatiles and water without inducing unwanted thermal degradation or phase changes in the fluoride product.
Drying vs. Roasting
It is crucial to distinguish the drying oven from the muffle furnace used earlier in the process.
As noted in comparative processing steps, a muffle furnace operates at much higher temperatures (600–631°C) to facilitate the chemical reaction (fluorination roasting) between the waste and ammonium hydrogen fluoride.
The drying oven, conversely, is a lower-temperature unit used strictly for physical moisture removal after the chemical conversion is complete.
Common Pitfalls to Avoid
The Risk of Incomplete Drying
Reducing the drying time below the recommended 12-hour cycle is a common error that compromises the entire batch.
Surface dryness can be deceptive; moisture trapped within the crystal lattice or aggregate structure requires sustained heat to migrate to the surface and evaporate.
Temperature Management
While rare earth fluorides are generally stable, excessive heat in a drying oven is unnecessary and inefficient.
Unlike the high-temperature roasting required to convert insoluble oxides, drying requires only enough energy to evaporate water.
Drastically increasing the temperature to "speed up" the process risks agglomerating the particles, similar to issues seen in other precursor drying processes, which can hinder subsequent handling or dissolution.
Making the Right Choice for Your Goal
To ensure the success of your rare earth recovery project, consider your immediate objective:
- If your primary focus is Molten Salt Electrolysis: Prioritize the duration of the drying cycle to ensure absolute dehydration, as even trace moisture will introduce oxygen impurities that degrade the final metal quality.
- If your primary focus is Material Characterization: Ensure the temperature remains stable at 80°C to provide a pristine, dry sample that yields accurate baseline data for X-ray diffraction and composition analysis.
Proper drying is the invisible barrier that protects the chemical integrity of your recovered rare earth products.
Summary Table:
| Parameter | Drying Oven Requirement | Significance |
|---|---|---|
| Temperature | 80°C (Constant) | Removes moisture without phase degradation |
| Duration | 12 Hours | Ensures complete dehydration of crystal lattice |
| Primary Goal | Moisture Removal | Prevents oxygen impurities in electrolysis |
| Secondary Goal | Sample Preparation | Guarantees precision in XRD and chemical analysis |
Optimize Your Rare Earth Recovery with KINTEK
Precision is non-negotiable when processing NdFeB waste. KINTEK provides high-performance laboratory drying ovens and specialized high-temp furnaces designed to safeguard your materials against chemical contamination.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to meet your unique thermal processing needs. Whether you are performing low-temperature dehydration or high-temperature fluorination roasting, our equipment ensures the chemical integrity of your rare earth products.
Ready to enhance your lab's efficiency and product purity?
Contact KINTEK Today for a Custom Solution
Visual Guide
References
- Optimization of Rare Earth Yield from Fluoride Roasting of Neodymium–Iron–Boron Waste Using Response Surface Methodology. DOI: 10.3390/met15090942
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1200℃ Muffle Oven Furnace for Laboratory
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- How does nano-MgO particle size influence sulfur doping in activated carbon? Optimize Doping for High-Performance Lab Materials
- How does controlled thermal treatment affect delta-MnO2? Optimize Porosity & Surface Area for Better Battery Performance
- What is the primary function of a laboratory blast drying oven? Essential Prep for La-EPS-C-450 Ceramic Adsorbents
- How does diamond benefit 5G technology? Unlock Peak Performance with Superior Thermal Management
- What is the firing temperature for sintering? A Guide to Material-Specific Ranges
- What is the significance of rapid quenching equipment in verifying the reaction pathway of BiFeO3? Capturing Intermediate Phases
- What is the maximum temperature capability of the furnace? Find Your Perfect High-Temp Solution
- Why is crushed glass used as a sealing agent during siliconization? Optimize Your High-Temp Reaction Purity



















