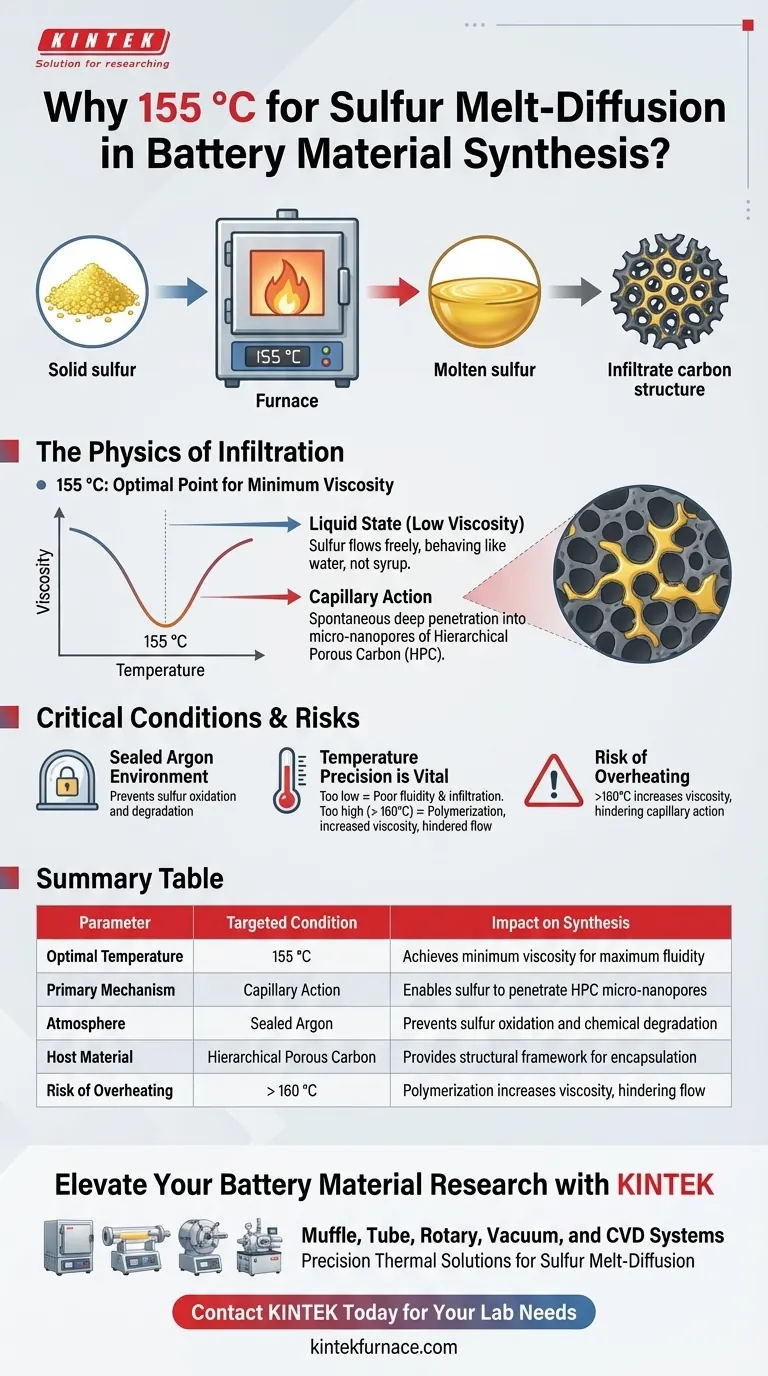
The temperature of 155 °C is chosen specifically to minimize the viscosity of molten sulfur. At this precise thermal point—which sits slightly above sulfur’s melting threshold—the material exhibits exceptional fluidity. This liquid state allows the sulfur to flow freely, enabling it to infiltrate complex carbon structures that would otherwise be inaccessible.
By maintaining the furnace at 155 °C, you create the optimal conditions for capillary action. In this state of minimum viscosity, molten sulfur can spontaneously and efficiently penetrate the micro-nanopores of Hierarchical Porous Carbon (HPC), ensuring a deep and uniform encapsulation of the active material.

The Physics of Sulfur Infiltration
Optimizing Fluidity
The primary goal of the melt-diffusion technique is to move solid sulfur into a porous host. At 155 °C, sulfur transforms into a liquid with extremely low viscosity.
This physical state is critical because the sulfur must behave more like water than a thick syrup. High fluidity ensures that the sulfur does not merely coat the surface of the carbon host but actually flows into it.
Leveraging Capillary Action
Once the sulfur achieves this low-viscosity state, it relies on capillary action to move.
This natural force draws the liquid sulfur into the microscopic voids of the Hierarchical Porous Carbon (HPC). Without the low viscosity achieved at 155 °C, capillary forces would be insufficient to pull the sulfur deep into the smallest micro-nanopores.
The Role of the Sealed Environment
This process is conducted in a sealed Argon environment.
Because sulfur is reactive and prone to oxidation at high temperatures, the inert argon atmosphere protects the chemical integrity of the materials. It ensures that the interaction remains purely physical (infiltration) rather than chemical (degradation) during the heating phase.
Understanding the Trade-offs
Temperature Precision is Vital
While 155 °C is the target, deviation from this temperature can compromise the synthesis.
If the temperature drops too low (closer to the melting point), the sulfur may not achieve the necessary fluidity to penetrate the deepest pores. This results in poor contact between the sulfur and the carbon host, reducing battery performance.
The Viscosity Risk at Higher Temperatures
It is critical not to significantly overshoot 155 °C.
While the primary reference highlights 155 °C for its low viscosity, it is important to note that sulfur’s viscosity does not decrease linearly with heat indefinitely. Overheating can alter the sulfur's molecular structure, potentially increasing viscosity and hindering the very capillary action you are trying to induce.
Making the Right Choice for Your Synthesis
To maximize the efficiency of your sulfur melt-diffusion process, focus on these operational priorities:
- If your primary focus is deep pore filling: Ensure your furnace creates a uniform 155 °C zone to maintain minimum viscosity throughout the entire soaking period.
- If your primary focus is material purity: Rigorously check your Argon seal, as the high fluidity of sulfur at this temperature increases its surface area and susceptibility to oxidation if leaks occur.
Mastering the melt-diffusion technique requires trusting the physics of viscosity to let the sulfur do the work for you.
Summary Table:
| Parameter | Targeted Condition | Impact on Synthesis |
|---|---|---|
| Optimal Temperature | 155 °C | Achieves minimum viscosity for maximum fluidity |
| Primary Mechanism | Capillary Action | Enables sulfur to penetrate HPC micro-nanopores |
| Atmosphere | Sealed Argon | Prevents sulfur oxidation and chemical degradation |
| Host Material | Hierarchical Porous Carbon | Provides the structural framework for encapsulation |
| Risk of Overheating | > 160 °C | Polymerization increases viscosity, hindering flow |
Elevate Your Battery Material Research with KINTEK
Precision is the difference between a high-performance battery and a failed synthesis. KINTEK provides the advanced thermal solutions necessary to master the sulfur melt-diffusion technique. Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable to meet your specific temperature uniformity and inert atmosphere requirements.
Don't let temperature fluctuations compromise your capillary infiltration. Contact KINTEK today to discuss your unique laboratory needs and discover how our high-precision furnaces can ensure your active materials are perfectly encapsulated every time.
Visual Guide
References
- Arunakumari Nulu, Keun Yong Sohn. N-doped CNTs wrapped sulfur-loaded hierarchical porous carbon cathode for Li–sulfur battery studies. DOI: 10.1039/d3ra08507d
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why is it necessary for sintering equipment to have a high-cooling-rate control for 17-4 PH? Master Your Metallurgy
- What is the significance of using a vacuum drying oven? Optimize Supercapacitor Electrode Performance
- What is the function of a laboratory vacuum drying oven for Fe-N-C catalysts? Preserve Nanoporous Structure
- What industries commonly use batch furnaces? Essential for Aerospace, Medical, and Electronics
- Why Use a Vacuum Oven for Cu-Cu2O/g-C3N4 Catalysts? Preserve Purity and Structural Integrity
- How does zinc chloride (ZnCl2) serve as a structural template? Engineering High-Porosity Nitrogen-Doped Carbon
- How does a continuous furnace differ from a batch furnace? Optimize Your Heat Treatment Process
- Why is it necessary to use an annealing furnace at 350°C for three hours? Ensuring Glass Stability and Clarity



















