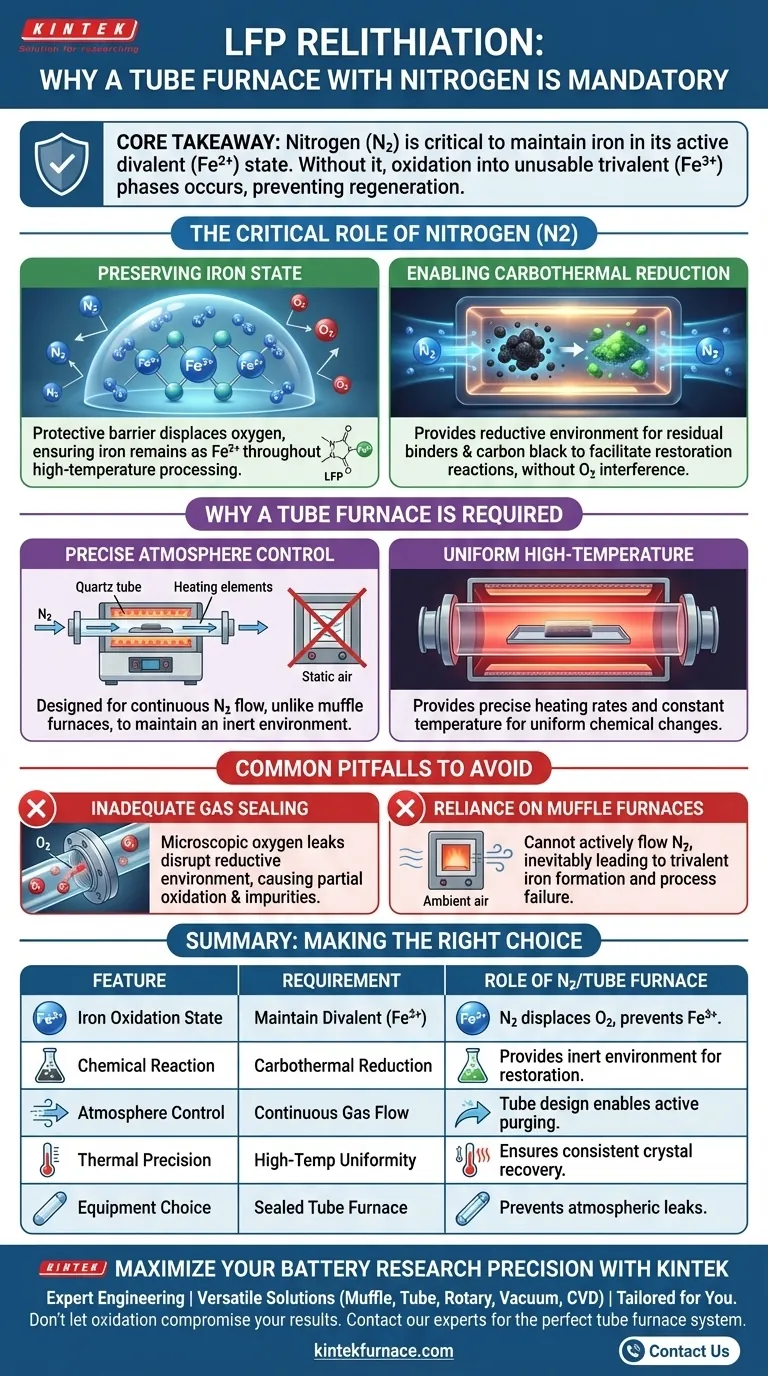
The use of a nitrogen (N2) atmosphere within a laboratory tube furnace is mandatory during the relithiation of Lithium Iron Phosphate (LFP) to create a strictly inert and reductive environment. This specific setup prevents the oxidation of the material, ensuring the iron remains in its required chemical state while enabling essential restoration reactions.
Core Takeaway The presence of nitrogen is critical to maintain iron in its active divalent (Fe2+) state. Without this protective atmosphere, the material oxidizes into unusable trivalent phases, preventing the successful regeneration of the battery material.

The Critical Role of the Nitrogen Atmosphere
Preserving the Iron State
The defining characteristic of LFP chemistry is the presence of iron in a divalent state (Fe2+).
During high-temperature processing, this state is highly susceptible to oxidation. If exposed to oxygen, Fe2+ transforms into trivalent iron (Fe3+), which degrades the electrochemical performance of the material.
A pure nitrogen atmosphere acts as a protective barrier. It displaces oxygen, ensuring the iron remains in the correct oxidation state throughout the heating process.
Enabling Carbothermal Reduction
Relithiation is not just about heating; it involves specific chemical reactions.
The process often utilizes residual binders and carbon black found in the spent material. These components facilitate carbothermal reduction reactions.
Nitrogen provides the necessary reductive environment for these reactions to occur. This allows the carbon to do its work effectively, aiding in the restoration of the crystal structure without competing with atmospheric oxygen.
Why a Tube Furnace is Required
Precise Atmosphere Control
Not all furnaces can maintain a specific gas environment.
Unlike muffle furnaces, which typically heat ambient air, tube furnaces are designed to pass gases through the heating chamber.
This capability allows for the continuous flow of nitrogen, ensuring the environment remains inert from start to finish. This flow control is similar to setups used in chemical vapor deposition (CVD).
Uniform High-Temperature Environment
Relithiation requires exact thermal conditions to be successful.
A laboratory tube furnace provides precise heating rates. It maintains a constant high-temperature environment, which is necessary to drive the chemical changes uniformly across the sample.
Common Pitfalls to Avoid
Inadequate Gas Sealing
The most common failure point is a leak in the tube furnace assembly.
Even a microscopic amount of oxygen entering the tube can disrupt the reductive environment. This leads to partial oxidation of the iron, resulting in impurities in the final LFP powder.
Reliance on Muffle Furnaces
Attempting this process in a standard muffle furnace is a frequent error.
Without the ability to actively flow nitrogen through the chamber, the atmosphere cannot be controlled. This inevitably results in the formation of trivalent iron phases, rendering the relithiation process a failure.
Making the Right Choice for Your Process
To ensure the successful regeneration of LFP material, apply the following guidelines:
- If your primary focus is Chemical Purity: Ensure your nitrogen source is high-purity and the gas flow rate is sufficient to flush all oxygen from the tube prior to heating.
- If your primary focus is Equipment Selection: Verify that your furnace is a tube design capable of active gas flow control, rather than a static muffle furnace.
Control the atmosphere effectively, and you protect the chemistry that powers the battery.
Summary Table:
| Feature | Requirement for LFP Relithiation | Role of Nitrogen/Tube Furnace |
|---|---|---|
| Iron Oxidation State | Must maintain Divalent (Fe2+) | N2 displaces oxygen to prevent trivalent (Fe3+) formation |
| Chemical Reaction | Carbothermal Reduction | Provides the inert environment for carbon-driven restoration |
| Atmosphere Control | Continuous Gas Flow | Tube furnace design enables precise and active gas purging |
| Thermal Precision | High-Temperature Uniformity | Ensures consistent crystal structure recovery across samples |
| Equipment Choice | Sealed Tube Furnace | Prevents atmospheric leaks that occur in standard muffle furnaces |
Maximize Your Battery Research Precision with KINTEK
Successful LFP relithiation demands absolute control over thermal and atmospheric variables. At KINTEK, we specialize in providing high-performance laboratory solutions tailored for advanced material science.
Why choose KINTEK?
- Expert Engineering: Backed by industry-leading R&D and manufacturing.
- Versatile Solutions: We offer specialized Muffle, Tube, Rotary, Vacuum, and CVD systems.
- Tailored for You: All high-temp furnaces are fully customizable to meet your specific gas flow and temperature profile requirements.
Don't let oxidation compromise your results. Contact our experts today to find the perfect tube furnace system for your lithium-ion battery research and manufacturing needs.
Visual Guide
References
- Elizabeth H. Driscoll, Emma Kendrick. Grave to Cradle: A Direct Recycling Approach for Over‐Discharged LiFePO<sub>4</sub> Electric Vehicle Cells. DOI: 10.1002/aesr.202500174
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What physical conditions does a high-temperature tube furnace provide? Optimize Lignin Carbonization Success
- How does the strong process performance of vacuum tube furnaces benefit users? Unlock Superior Quality and Efficiency
- Why is a controlled nitrogen atmosphere necessary within a tube furnace during the annealing of Antimony-doped thin films?
- How does a tube vacuum furnace ensure quality during the solution treatment of aluminum matrix composites? Unlock Precision and Purity for Superior Materials
- Why is a high-precision tube furnace required during Fe-Mn catalyst synthesis? Control Morphology and CNF Quality
- How does high-temperature annealing in a tube furnace influence the performance of the RuCuCl/NF-2 catalyst?
- What role does a high-temperature tube furnace play in POLO contact structures? Unlock High-Efficiency Silicon Contacts
- How do furnace chamber working conditions influence the choice of a tube furnace? Optimize Performance and Cost



















