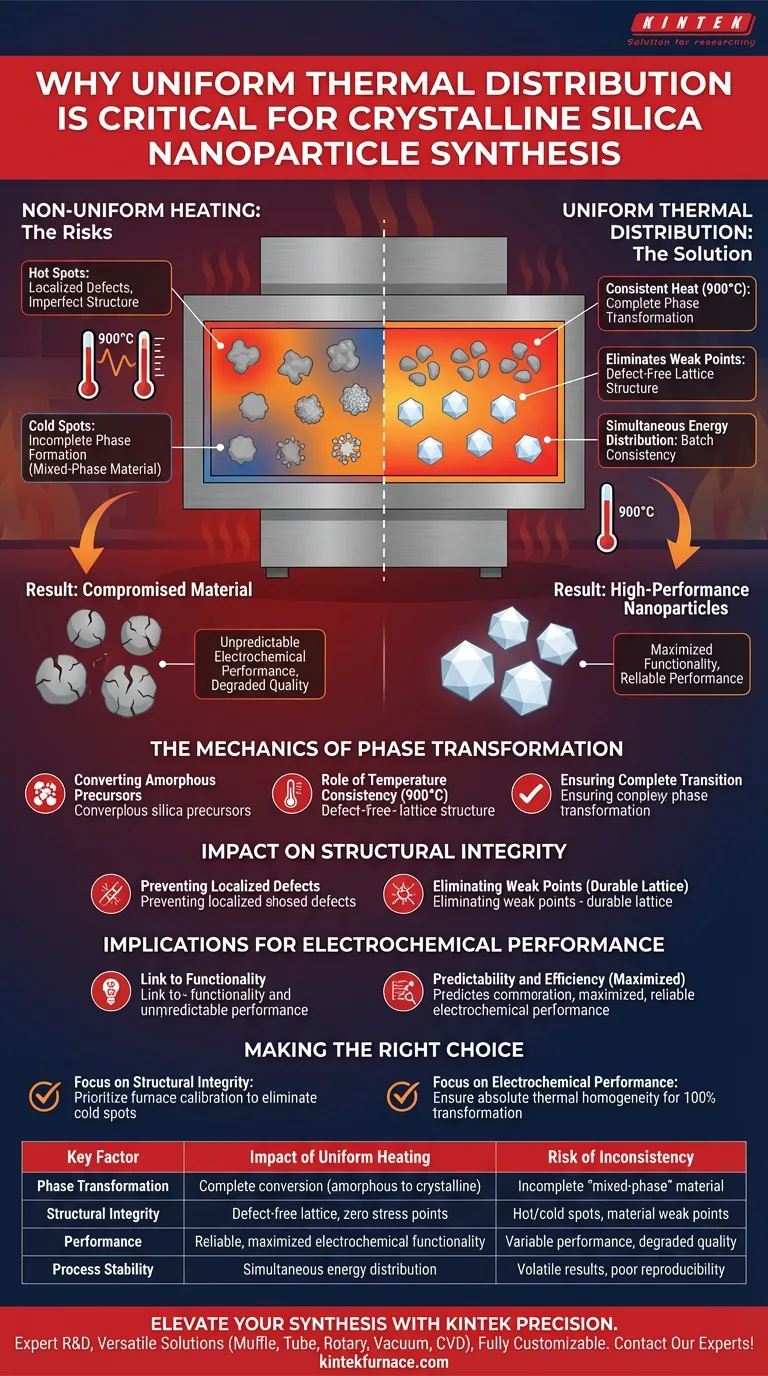
Achieving uniform thermal distribution is the definitive factor in the successful synthesis of crystalline silica nanoparticles because it guarantees consistent phase transformation across the entire material volume. Without precise thermal homogeneity, particularly at critical temperatures like 900°C, the amorphous precursor fails to transition evenly, resulting in a compromised material with unpredictable properties.
Uniform heating is not merely about temperature control; it is the specific mechanism that prevents localized defects and ensures the material transitions fully from an amorphous state to a stable crystalline structure. This consistency is the prerequisite for structural integrity and reliable electrochemical performance.

The Mechanics of Phase Transformation
Converting Amorphous Precursors
The synthesis process relies on converting an amorphous silica precursor into a structured crystalline form, such as cristobalite.
The Role of Temperature Consistency
This transformation requires sustained exposure to high temperatures, specifically around 900°C. If the heat is applied unevenly, the energy required to trigger this phase change is not distributed equally.
Ensuring Complete Transition
Uniform distribution ensures that every nanoparticle within the batch receives the necessary energy to cross the crystallization threshold simultaneously.
Impact on Structural Integrity
Preventing Localized Defects
When thermal distribution varies, it leads to "hot spots" or "cold spots" within the furnace. These variances cause localized defects where the crystal structure is either imperfect or incomplete.
Eliminating Weak Points
A uniform thermal environment prevents stress points within the material. This ensures that the final nanoparticles possess a consistent, defect-free lattice structure vital for durability.
Implications for Electrochemical Performance
The Link to Functionality
For high-performance applications, the physical structure of the nanoparticle dictates its electrochemical behavior.
Predictability and Efficiency
Inconsistencies in heating lead to variations in crystallinity. This results in a material that performs unpredictably. Uniform heating ensures that the electrochemical properties are maximized and consistent throughout the entire batch.
The Risks of Thermal Inconsistency
Incomplete Phase Formation
The primary pitfall of non-uniform heating is the production of a "mixed-phase" material. Some areas may fully crystallize into cristobalite, while others remain partially amorphous.
Compromised Material Quality
This lack of homogeneity degrades the overall quality of the synthesis. It renders the material unsuitable for applications where high structural integrity and specific electrochemical traits are non-negotiable.
Making the Right Choice for Your Goal
To optimize your synthesis process, focus on how thermal distribution aligns with your specific material requirements.
- If your primary focus is structural integrity: Prioritize furnace calibration to eliminate cold spots, ensuring no part of the material volume suffers from localized lattice defects.
- If your primary focus is electrochemical performance: Ensure absolute thermal homogeneity at 900°C to guarantee a 100% complete phase transformation from amorphous to crystalline.
Mastering thermal distribution is the only way to turn a volatile precursor into a reliable, high-performance nanoparticle.
Summary Table:
| Key Factor | Impact of Uniform Heating | Risk of Inconsistency |
|---|---|---|
| Phase Transformation | Complete conversion from amorphous to crystalline (e.g., cristobalite) | Incomplete "mixed-phase" material with unpredictable traits |
| Structural Integrity | Defect-free lattice structure with zero localized stress points | Hot/cold spots leading to material weak points and defects |
| Performance | Reliable and maximized electrochemical functionality | Variable performance and degraded material quality |
| Process Stability | Simultaneous energy distribution across the entire batch | Volatile results and poor batch-to-batch reproducibility |
Elevate Your Material Synthesis with KINTEK Precision
Don't let thermal inconsistency compromise your research outcomes. At KINTEK, we understand that 100% phase transformation requires absolute thermal homogeneity. Our high-temperature furnace solutions are designed to eliminate hot spots and ensure stable, uniform heating for critical processes like silica nanoparticle synthesis.
Why choose KINTEK?
- Expert R&D & Manufacturing: Precision-engineered systems tailored for high-tech applications.
- Versatile Solutions: From Muffle and Tube Furnaces to Rotary, Vacuum, and CVD systems.
- Fully Customizable: Equipment built to meet your unique temperature and atmospheric requirements.
Ensure the structural integrity of your materials today. Contact our experts now to find the perfect furnace for your lab!
Visual Guide
References
- Sohan Thombare, C.D. Lokhande. Synthesis and characterization of crystalline cristobalite alpha low silicon dioxide nanoparticles: a cost-effective anode for lithium-ion battery. DOI: 10.1007/s10854-024-13153-8
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What are the advantages of a multimode microwave furnace? Accelerate B-doped SiC Nanowire Synthesis for Higher Yields
- What is the purpose of silver paste coating for BCZT ceramics? Ensuring Precision in Electrical Performance Testing
- Why is precise temperature control necessary for drying plum stone raw materials? Enhance Biochar Quality & Grinding
- Why is the extrusion temperature for PVC biocomposites typically set at 130°C? Achieve Perfect Thermal Balance
- What are the advantages of continuous furnaces? Boost Efficiency and Cut Costs in High-Volume Production
- What role does an industrial fast firing furnace play in the metallization of PERT solar cells? Boost Cell Efficiency
- Why is a two-stage heat treatment required for Ca2Fe2O5? Optimize Your Brownmillerite Synthesis
- Why is the laboratory heating and boiling stage essential in the maceration process of wood fibers?



















