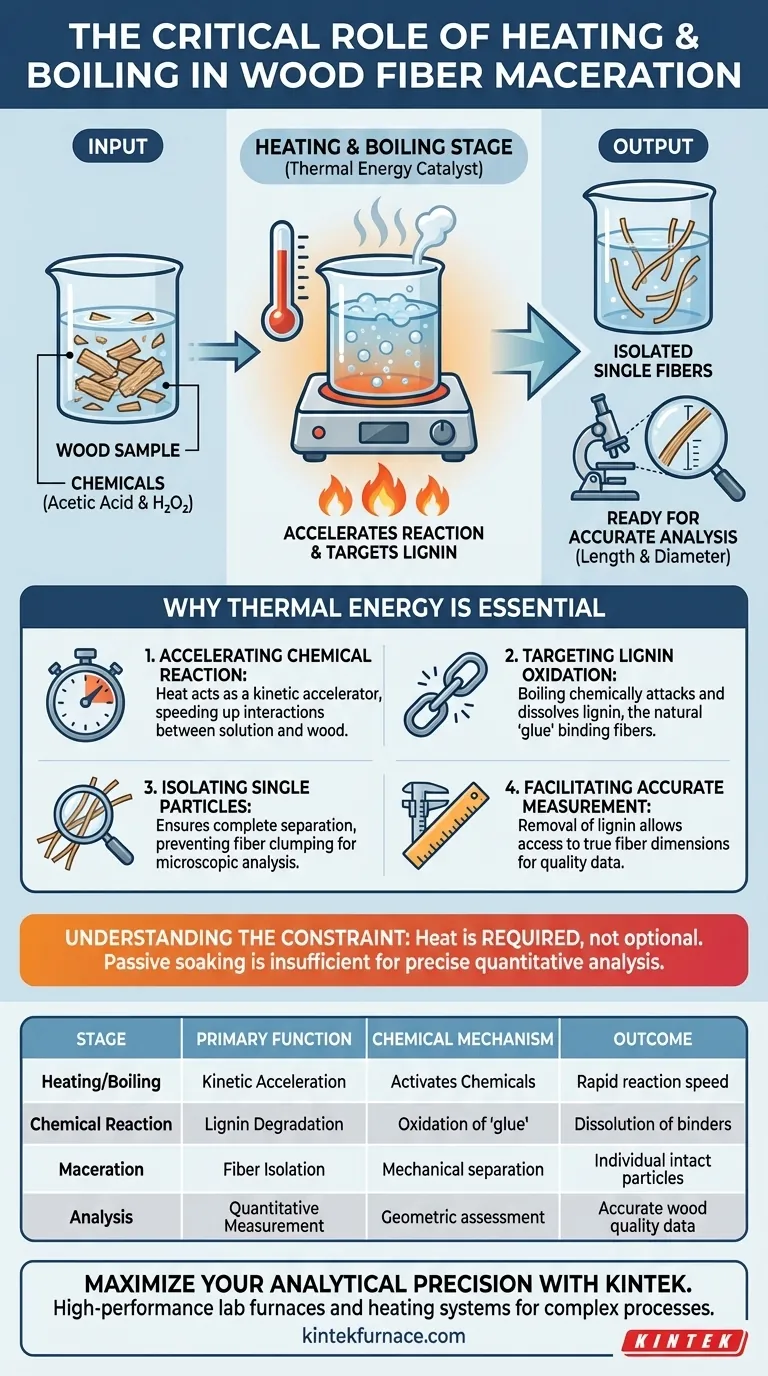
The laboratory heating and boiling stage is the critical catalyst that drives the chemical separation of wood fibers. By applying thermal energy to a mixture of glacial acetic acid and hydrogen peroxide, the process significantly accelerates the oxidation and degradation of lignin. This rapid breakdown of the wood's internal binding structure is essential to isolate individual fibers for analysis.
Without the application of sustained heat, the chemical agents in the maceration process would fail to dissolve the lignin binder efficiently. Boiling provides the necessary thermal energy to separate fibers cleanly, ensuring their length and diameter can be measured accurately as intact single particles.

The Role of Thermal Energy in Maceration
Accelerating the Chemical Reaction
In methods such as the Franklin method, the chemical solution alone is often insufficient to break down wood structure in a timely manner. The mixture of glacial acetic acid and hydrogen peroxide requires activation.
The boiling stage introduces high thermal energy into this mixture. This energy acts as a kinetic accelerator, speeding up the chemical interactions between the solution and the wood sample.
Targeting Lignin Oxidation
The primary obstacle to fiber separation is lignin, the natural "glue" that holds wood cells together.
The heating process specifically drives the oxidation and degradation of lignin. As the solution boils, it chemically attacks the lignin structure, causing it to dissolve and release its hold on the cellulose fibers.
Ensuring Analytical Precision
Isolating Single Particles
The ultimate goal of laboratory maceration is not just to break down wood, but to obtain intact single fiber particles.
If the lignin is not fully degraded through boiling, the fibers will remain clumped in bundles. By ensuring complete separation, the heating stage allows for the isolation of individual strands necessary for microscopic analysis.
Facilitating Accurate Measurement
Once the fibers are separated, they must be analyzed for specific geometric properties.
The removal of lignin allows researchers to access the true dimensions of the fiber. This is strictly necessary for the precise measurement of fiber length and diameter, which are key indicators of wood quality and utility.
Understanding Process Constraints
The Necessity of Active Energy
It is important to recognize that passive soaking is rarely sufficient for this type of quantitative analysis.
The reference highlights that boiling is required, not optional. Omitting the heating stage would likely result in incomplete maceration, leaving fibers attached and rendering precise dimensional measurement impossible.
Making the Right Choice for Your Goal
To ensure your maceration process yields usable data, consider the following based on your objectives:
- If your primary focus is process efficiency: Ensure the mixture reaches a boil to maximize the reaction speed of the acetic acid and hydrogen peroxide.
- If your primary focus is data accuracy: Maintain the heating stage until lignin is fully degraded to guarantee the extraction of intact, measurable single fibers.
The thermal input is the deciding factor that transforms a solid wood sample into an analyzable suspension of individual structural elements.
Summary Table:
| Stage | Primary Function | Chemical Mechanism | Outcome |
|---|---|---|---|
| Heating/Boiling | Kinetic Acceleration | Activates Acetic Acid & Hydrogen Peroxide | Rapid reaction speed |
| Chemical Reaction | Lignin Degradation | Oxidation of the lignin "glue" | Dissolution of cell binders |
| Maceration | Fiber Isolation | Mechanical separation of cell walls | Individual intact particles |
| Analysis | Quantitative Measurement | Geometric assessment (Length/Diameter) | Accurate wood quality data |
Maximize Your Analytical Precision with KINTEK
Precise fiber isolation requires consistent, controlled thermal energy. KINTEK provides high-performance lab furnaces and heating systems designed to facilitate complex chemical processes like wood maceration. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique research specifications.
Ready to enhance your lab’s efficiency and data accuracy? Contact KINTEK today to find your custom thermal solution!
Visual Guide
References
- Issah Chakurah, Enoch Gbapenuo Tampori. The Effect of Thermal Modification on Anatomical Properties of Daniellia oliveri (Rolfe) Hutch and Dalziel from Ghana. DOI: 10.5552/drvind.2025.0218
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does a plasma surface metallurgy furnace achieve infiltration? Engineer High-Performance Diffusion Bonds
- What is quenching, and why is it important? Achieve Superior Material Hardness and Strength
- What are the advantages of ascorbic acid over glucose in LFP synthesis? Achieve Superior Purity and Crystallinity
- What is the purpose of sintering furnaces? Transform Powders into Strong, Dense Materials
- How does a high-precision reaction system aid methane CLR research? Unlock Advanced Syngas Insights
- What role does a pyrolysis furnace play in preparing graphene nanosheets? Master High-Value Plastic Transformation
- What factors are assessed during the evaluation for an Industrial Furnace project? Optimize Your Thermal Process
- What role does a high-frequency LCR meter play in analyzing the CIS of SSBSN ceramics? Unlocking Microstructural Secrets



















