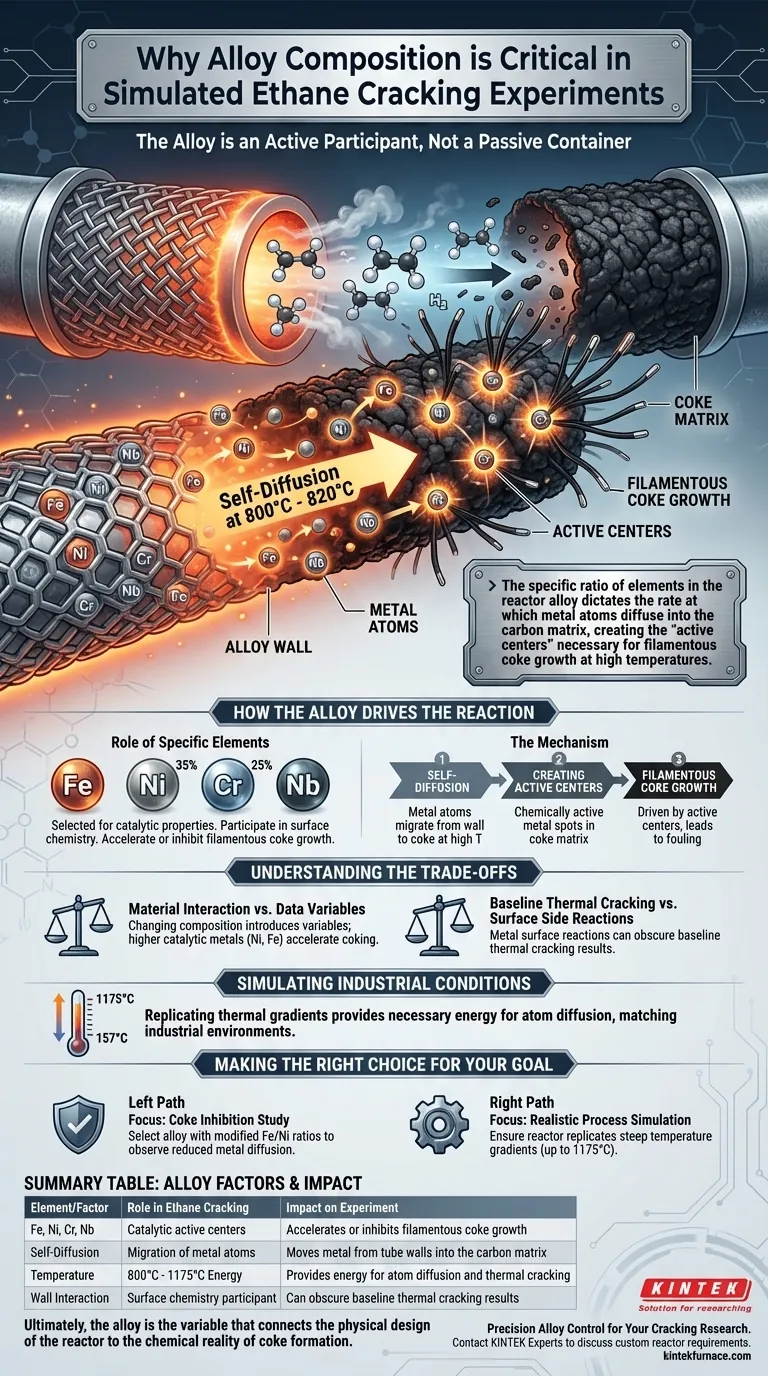
The chemical composition of the alloy is not a passive container; it is an active participant in the reaction. In simulated ethane cracking, specific elements—such as iron, nickel, chromium, and niobium—act as catalysts rather than mere structural components. Researchers meticulously control this composition to study how metal atoms migrate from the tube walls to induce and sustain the formation of coke.
The specific ratio of elements in the reactor alloy dictates the rate at which metal atoms diffuse into the carbon matrix, creating the "active centers" necessary for filamentous coke growth at high temperatures.

How the Alloy Drives the Reaction
Beyond Structural Integrity
In standard engineering, alloys are chosen for strength and heat resistance. In ethane cracking experiments, however, the alloy plays a chemical role. The reactor walls interact directly with the process gas.
The Role of Specific Elements
Common compositions, such as those containing varying weights of iron, nickel (e.g., 35%), chromium (e.g., 25%), and niobium, are selected for their catalytic properties. These metals do not remain static; they participate in the surface chemistry that occurs during cracking.
The Mechanism of Coke Formation
Self-Diffusion of Atoms
At operating temperatures between 800°C and 820°C, a critical physical phenomenon occurs: self-diffusion. Metal atoms from the alloy lattice migrate (diffuse) outwards. They move from the solid tube wall into the developing layer of coke (carbon deposits).
Creating Active Centers
This diffusion is not random; it creates metal "active centers" within the coke matrix. These centers are chemically active spots that facilitate further reaction.
Filamentous Coke Growth
The presence of these metal active centers is the primary driver for a specific type of fouling called filamentous coke. By controlling the alloy composition, researchers can accelerate or inhibit this growth to understand the underlying kinetics.
Understanding the Trade-offs
The Complexity of Material Interaction
While changing the alloy composition provides valuable data on coke formation, it introduces variables that must be carefully managed. A higher concentration of catalytic metals (like nickel or iron) may accelerate coking mechanisms.
Distinguishing Reaction Types
This acceleration can sometimes obscure the baseline thermal cracking results. Researchers must differentiate between the cracking caused by heat and the side reactions caused by the metal surface itself.
Simulating Industrial Conditions
Replicating Thermal Gradients
To make these findings applicable to real-world plants, the physical environment must match the chemical one. Tubular reactors use heating zones to create massive temperature gradients, often ranging from 1175°C down to 157°C.
Physical Space for Diffusion
These thermal conditions provide the necessary energy for the metal diffusion described above. The reactor design ensures that the thermodynamic conditions inside the tube mimic the harsh environment of industrial production.
Making the Right Choice for Your Goal
To optimize your experimental setup, you must align the alloy selection with your specific research objective.
- If your primary focus is studying coke inhibition: Select alloy compositions with modified iron or nickel ratios to observe how reduced metal diffusion slows filamentous growth.
- If your primary focus is realistic process simulation: Ensure your reactor replicates the steep temperature gradients (up to 1175°C) to validate that the alloy behaves thermodynamically as it would in a commercial plant.
Ultimately, the alloy is the variable that connects the physical design of the reactor to the chemical reality of coke formation.
Summary Table:
| Element/Factor | Role in Ethane Cracking | Impact on Experiment |
|---|---|---|
| Fe, Ni, Cr, Nb | Catalytic active centers | Accelerates or inhibits filamentous coke growth |
| Self-Diffusion | Migration of metal atoms | Moves metal from tube walls into the carbon matrix |
| Temperature | 800°C - 1175°C | Provides energy for atom diffusion and thermal cracking |
| Wall Interaction | Surface chemistry participant | Can obscure baseline thermal cracking results |
Precision Alloy Control for Your Cracking Research
Understanding the complex interplay between reactor metallurgy and coke formation is essential for accurate laboratory simulations. KINTEK provides high-performance, customizable thermal solutions designed to meet the rigorous demands of chemical research. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems tailored to your unique experimental needs.
Whether you are studying coke inhibition kinetics or replicating industrial thermal gradients, our team delivers the specialized high-temperature furnaces required for reliable data.
Optimize your experimental results today — Contact KINTEK Experts to discuss your custom reactor requirements.
Visual Guide
References
- P. Nanthagopal R. Sachithananthan. Analytical Review on Impact of Catalytic Coke Formation on Reactor Surfaces During the Thermal Cracking Process. DOI: 10.5281/zenodo.17985551
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
People Also Ask
- What role does a laboratory tube furnace play in the carbonization process of porous carbon particles? Expert Insights
- How do roller kilns and tube furnaces differ in their use of Alumina ceramic tubes? Compare Transport vs. Containment
- What is the function of a Tube Furnace during molybdenum carbide synthesis? Master Catalyst Carbonization
- How to clean a tubular furnace? A Step-by-Step Guide to Safe and Effective Maintenance
- What factors should be considered when selecting a horizontal electric furnace? Ensure Precision and Efficiency for Your Lab
- What is the purpose of introducing nitrogen flow into a tube furnace? Optimize Your Activated Carbon Calcination
- What critical conditions does a vacuum tube furnace provide for superconductor Tc measurement? Precision thermal control
- What technical challenges are associated with tube furnace cracking? Master Extreme Heat and Material Limits



















