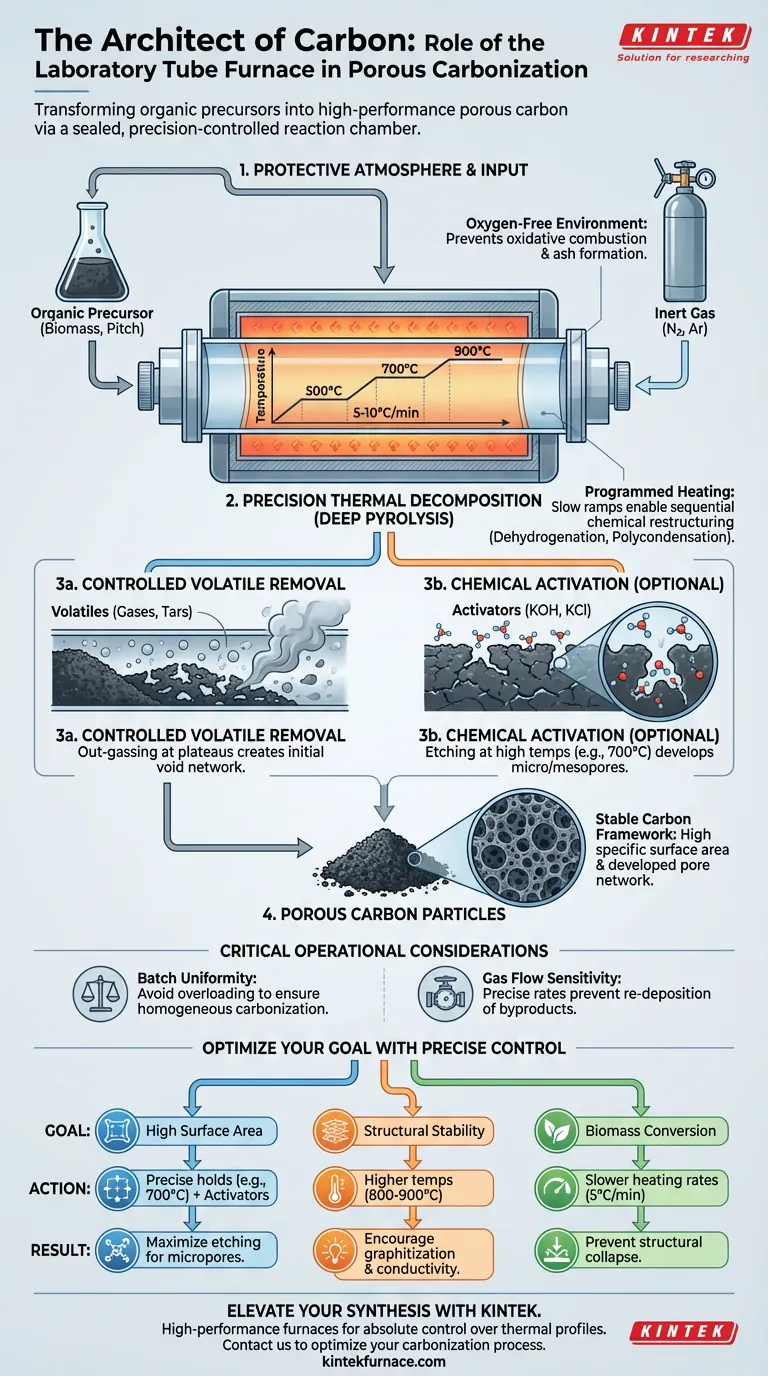
A laboratory tube furnace functions as the critical reaction chamber for transforming organic precursors into porous carbon particles. It provides a sealed, strictly controlled environment that enables high-temperature thermal treatment while preventing the material from burning away. By managing both the atmosphere and the heating profile, the furnace dictates the chemical evolution of the material from a raw solid into a stable carbon framework.
Core Takeaway: The tube furnace facilitates deep pyrolysis by maintaining a precise inert atmosphere (typically Nitrogen or Argon) and exact temperature gradients. This controlled environment prevents oxidative combustion, forcing the material to undergo dehydrogenation and devolatilization, which creates the essential void spaces that define porous carbon.
The Mechanics of Carbonization
Establishing the Protective Atmosphere
The most fundamental role of the tube furnace is atmospheric sealing. To create porous carbon, you must heat materials to extreme temperatures (often between 500°C and 900°C) without allowing them to combust.
The furnace creates an oxygen-free environment using inert gases like nitrogen or argon, or by establishing a vacuum. This prevents "oxidative ablation," ensuring the raw material converts into carbon rather than turning into ash.
Precision Thermal Decomposition
Carbonization is not merely heating; it is a complex chemical restructuring. The tube furnace executes programmed heating rates, typically slow ramps of 5 to 10°C per minute.
This slow, controlled rise allows for specific chemical reactions—such as dehydrogenation and polycondensation—to occur sequentially. Whether processing petroleum pitch or biomass, this precision ensures the carbon atoms rearrange into a stable lattice rather than fracturing randomly.
Driving Pore Formation and Structure
Controlled Volatile Removal
As the furnace holds temperatures at specific plateaus (e.g., 500°C or 600°C), it facilitates deep pyrolysis. This process drives out volatile components (gases and tars) trapped within the material.
The escape of these volatiles leaves behind vacancies in the material's structure. The furnace's stability ensures this "out-gassing" happens consistently, resulting in a developed network of pores and a high specific surface area.
Facilitating Chemical Activation
For advanced porous carbons, the furnace often works in tandem with chemical activators like KOH or KCl. By holding temperatures at precise points (e.g., 700°C), the furnace enables these chemicals to etch the carbon surface.
This etching process creates a rich microstructure of micropores and mesopores. The thermal stability of the furnace is vital here; fluctuations in temperature would lead to uneven etching and inconsistent pore size distributions.
Critical Operational Considerations
Batch Volume vs. Uniformity
While tube furnaces offer exceptional control, they are inherently limited by the size of the reaction tube. Overloading the tube can lead to thermal gradients where the center of the sample reaches a different temperature than the edges, resulting in heterogeneous carbonization.
Sensitivity to Gas Flow
The outcome of the carbonization is highly sensitive to the flow rate of the inert gas. If the flow is too low, volatile byproducts may re-deposit on the carbon surface, clogging the very pores you are trying to create. If too high, it may disturb the thermal equilibrium.
Making the Right Choice for Your Goal
To maximize the utility of a tube furnace for your specific carbon material, align your settings with your desired outcome:
- If your primary focus is high surface area: Prioritize precise temperature holds (e.g., 700°C) alongside chemical activators to maximize the etching effect.
- If your primary focus is structural stability (Graphitization): Utilize higher temperature capabilities (800°C - 900°C) to encourage atomic rearrangement and higher conductivity.
- If your primary focus is biomass conversion: Adhere to slower heating rates (5°C/min) to prevent structural collapse during the rapid release of volatiles.
The laboratory tube furnace is not just a heater; it is the architect of the carbon skeleton, determining the final porosity and performance of your material through rigid environmental control.
Summary Table:
| Feature | Role in Carbonization | Impact on Porous Carbon |
|---|---|---|
| Atmosphere Control | Prevents oxidative combustion via inert gases | Ensures conversion to carbon instead of ash |
| Thermal Precision | Programmed heating rates (5-10°C/min) | Facilitates stable atomic lattice rearrangement |
| Volatile Removal | Consistent out-gassing at plateaus | Creates the essential network of internal pores |
| Chemical Activation | Enables surface etching at high temps | Develops high specific surface area & micropores |
Elevate Your Material Synthesis with KINTEK
Precision is the difference between simple ash and high-performance porous carbon. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems designed to give you absolute control over your thermal profiles.
Whether you are processing biomass or advanced petroleum pitch, our customizable laboratory high-temp furnaces ensure the atmospheric purity and thermal stability your research demands.
Ready to optimize your carbonization process?
→ Contact Our Technical Specialists Today
Visual Guide
References
- Betül Ari, Nurettin Şahiner. Optimized Porous Carbon Particles from Sucrose and Their Polyethyleneimine Modifications for Enhanced CO2 Capture. DOI: 10.3390/jcs8090338
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- Why is a High Vacuum or High Purity Nitrogen Tube Furnace necessary for the growth of carbide precipitates in steel?
- Why is a high-temperature tube furnace required for CrFeNi alloy treatment? Ensure Single-Phase Microstructural Stability
- What role does atmosphere control play in tube furnace applications? Master Precise Chemical Reactions for Superior Materials
- What are the applications of a laboratory tube furnace in physics research? Unlock Precise High-Temperature Experiments
- What is the specific role of a tube furnace in the pre-treatment of activated carbon catalysts? Precision Modification
- What is the function of a tube furnace in pRF preparation? Optimize Carbonization & Conductivity
- What role do tubular furnaces play in heat treatment processes? Precision Control for Material Properties
- Why is a stable nitrogen flow required in a tube furnace for hydrochar carbonization? Ensure High-Carbon Purity



















