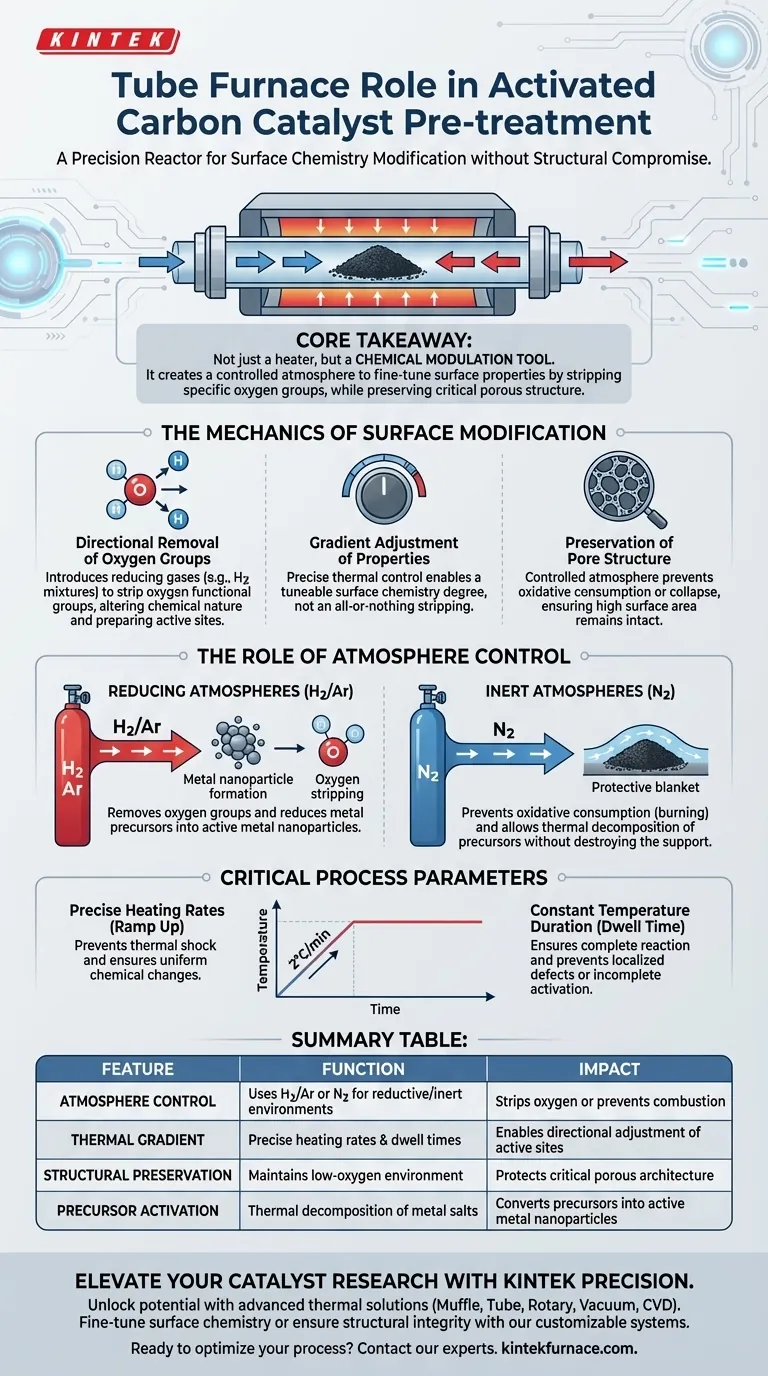
The tube furnace serves as a precision reactor designed to modify the surface chemistry of activated carbon without compromising its physical structure.
Specifically, it provides a controlled high-temperature environment that utilizes reducing gases (such as hydrogen mixtures) to systematically remove oxygen-containing functional groups. By strictly regulating heating rates and dwell times, the furnace allows for the "directional" adjustment of catalytic active sites while ensuring the material's porous architecture remains intact.
Core Takeaway The tube furnace is not merely a heating device; it is a chemical modulation tool. Its primary function in this context is to create a controlled, often reductive, atmosphere that fine-tunes the chemical properties of the carbon surface—stripping away specific oxygen groups—while preserving the critical surface area and pore structure required for catalysis.

The Mechanics of Surface Modification
Directional Removal of Oxygen Groups
The primary reference highlights that activated carbon surfaces often contain various oxygen-functional groups. The tube furnace facilitates the directional removal of these groups.
By introducing reducing gases, the furnace strips oxygen from the surface. This alters the chemical nature of the carbon, effectively preparing the "active sites" where future catalytic reactions will occur.
Gradient Adjustment of Properties
This process is not an all-or-nothing reaction. Through precise thermal control, the furnace enables a gradient adjustment of chemical properties.
This means you can tune the surface chemistry to a specific degree—rather than simply stripping everything away—by manipulating the temperature profile and gas concentration.
Preservation of Pore Structure
A critical requirement for activated carbon is high surface area. The tube furnace ensures that while the surface chemistry changes, the physical structure does not.
Unlike oxidative environments that might burn away the carbon or collapse the pores, the controlled atmosphere ensures the pore structure remains undamaged during treatment.
The Role of Atmosphere Control
Reducing Atmospheres (Hydrogen/Argon)
To remove oxygen groups or reduce metal precursors, the furnace typically utilizes hydrogen mixtures.
This creates a chemical environment where reduction occurs efficiently. As noted in supplementary data, this is also essential for reducing metal precursors supported on the material into active metal nanoparticles.
Inert Atmospheres (Nitrogen)
When the goal is to prevent oxidation, an inert nitrogen flow is employed.
This effectively blankets the activated carbon, preventing "oxidative consumption" (burning) of the substrate. It allows for thermal decomposition of precursors (like copper nitrate) without destroying the carbon support itself.
Critical Process Parameters
Precise Heating Rates
The "ramp up" speed is as important as the final temperature.
The tube furnace allows for specific heating rates (e.g., 2°C/min). This slow, controlled rise prevents thermal shock and ensures that chemical changes, such as ligand stripping or the formation of surface oxygen vacancies, occur uniformly.
Constant Temperature Duration
The "dwelling time" determines the extent of the reaction.
Maintaining a constant temperature for a set duration ensures that the removal of functional groups or the reduction of metal oxides is complete, preventing localized defects or incomplete activation.
Understanding the Trade-offs
Atmosphere Sensitivity
The effectiveness of a tube furnace is entirely dependent on the purity and flow of the gas atmosphere. Even trace amounts of oxygen in a reduction cycle can lead to the unintended combustion of the activated carbon substrate, destroying the sample.
Throughput Limitations
While excellent for precision, tube furnaces generally have lower throughput compared to industrial rotary kilns or fluidized beds. They are batch-process oriented, making them ideal for high-value, precise pre-treatment but potentially bottlenecked for mass production unless scaled horizontally.
Making the Right Choice for Your Goal
Depending on your specific catalytic requirements, you must adjust the tube furnace parameters accordingly:
- If your primary focus is Tuning Surface Acidity/Basicity: Prioritize the directional removal of oxygen groups using hydrogen mixtures to adjust the chemical state of the active sites without altering the pores.
- If your primary focus is Metal Precursor Activation: Focus on inert or reducing atmospheres to decompose salts (like nitrates) into oxides or metals while preventing the oxidative loss of the carbon support.
- If your primary focus is Structural Integrity: strictly limit the heating rate to prevent thermal shock and ensure the pore network remains open and accessible.
The tube furnace is the bridge between a raw material and a tuned catalyst, providing the exact atmospheric and thermal conditions required to engineer the surface at a molecular level.
Summary Table:
| Feature | Function in Catalyst Pre-treatment | Impact on Activated Carbon |
|---|---|---|
| Atmosphere Control | Uses H2/Ar or N2 to create reductive or inert environments | Strips oxygen groups or prevents oxidative combustion |
| Thermal Gradient | Precise heating rates (e.g., 2°C/min) and dwell times | Enables directional adjustment of chemical active sites |
| Structural Preservation | Maintains low-oxygen environment during heating | Protects the critical porous architecture and surface area |
| Precursor Activation | Thermal decomposition of metal salts (e.g., nitrates) | Converts precursors into active metal nanoparticles |
Elevate Your Catalyst Research with KINTEK Precision
Unlock the full potential of your materials with KINTEK’s advanced thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers customizable Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of lab-scale and industrial high-temperature processing.
Whether you need to fine-tune surface chemistry or ensure the structural integrity of your activated carbon supports, our furnaces provide the atmospheric purity and thermal stability your research deserves.
Ready to optimize your pre-treatment process? Contact our technical experts today to find the perfect system for your unique needs.
Visual Guide
References
- Xuhan Li, Liqiang Zhang. Boosting Hydrogen Production from Hydrogen Iodide Decomposition over Activated Carbon by Targeted Removal of Oxygen Functional Groups: Evidence from Experiments and DFT Calculations. DOI: 10.3390/en18164288
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- What safety measures are essential when operating a lab tube furnace? A Guide to Preventing Accidents
- What is an example of a material prepared using a tube furnace? Master Precise Material Synthesis
- How do vertical tube furnaces comply with environmental standards? A Guide to Clean, Efficient Operation
- What role does a laboratory tube furnace perform during the carbonization of LCNSs? Achieve 83.8% Efficiency
- What safety and reliability features are incorporated into a vertical tube furnace? Ensuring Safe, Consistent High-Temp Processing



















