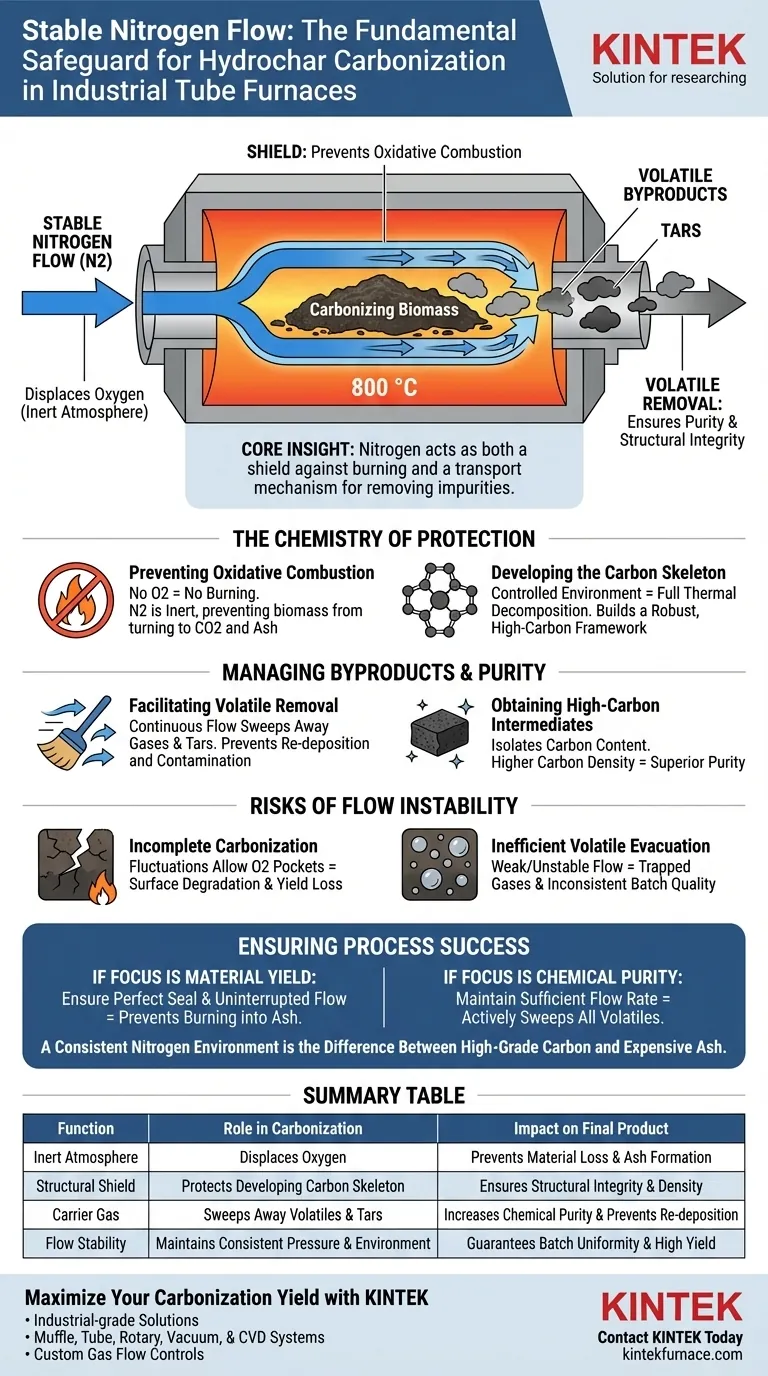
A stable nitrogen flow is the fundamental safeguard against material destruction during carbonization. In an industrial-grade tube furnace operating at 800 °C, this flow is required to displace oxygen and create an inert atmosphere. Without it, the biomass would undergo oxidative combustion (burning) rather than carbonization, resulting in ash instead of the desired high-carbon material.
Core Insight: Nitrogen acts as both a shield and a transport mechanism. It prevents the carbon structure from burning away while simultaneously sweeping away volatile byproducts to ensure the purity and structural integrity of the final carbon skeleton.

The Chemistry of Protection
To understand why nitrogen is non-negotiable, you must look at the chemical reaction occurring at 800 °C.
Preventing Oxidative Combustion
At high temperatures, carbon is highly reactive with oxygen. If air enters the furnace, the biomass will simply burn, converting valuable carbon into carbon dioxide and ash.
Nitrogen is an inert gas, meaning it does not react with the hydrochar. By flooding the chamber with nitrogen, you deny the process the oxygen required for combustion to occur.
Developing the Carbon Skeleton
The goal of carbonization is to rearrange the internal structure of the biomass. This requires a controlled environment where the material can decompose thermally without chemical interference.
A stable nitrogen atmosphere allows the carbon material skeleton to develop fully and naturally. This ensures the structural framework remains intact, serving as the foundation for the final product's physical properties.
Managing Byproducts and Purity
Beyond protection, the flow of nitrogen plays an active mechanical role in the quality of the output.
Facilitating Volatile Removal
As the hydrochar heats up, it releases volatile components (gases and tars). If these volatiles remain stagnant around the sample, they can re-deposit or interfere with the surface chemistry.
The flow of nitrogen—not just its presence—acts as a carrier mechanism. It continuously sweeps these volatile components out of the heating zone, preventing contamination.
Obtaining High-Carbon Intermediates
The ultimate objective is to isolate the carbon content. By preventing oxidation and removing non-carbon volatiles, nitrogen ensures the remaining material is a high-carbon intermediate.
This results in a purer product with a higher carbon density, which is the primary metric of success for this process.
The Risks of Flow Instability
While the presence of nitrogen is required, the stability of that flow is equally critical.
Incomplete Carbonization
If the flow fluctuates or drops, pockets of oxygen may enter the system. Even a momentary lapse at 800 °C can degrade the surface of the material, leading to a loss of yield.
Inefficient Volatile Evacuation
If the flow is too weak or unstable, volatiles may not be removed efficiently. This can lead to inconsistent quality across the batch, as trapped gases affect the final development of the carbon structure.
Ensuring Process Success
To maximize the quality of your hydrochar, focus on the stability and consistency of your inert gas system.
- If your primary focus is Material Yield: Ensure the furnace is perfectly sealed and the nitrogen flow is uninterrupted to prevent the biomass from burning into ash.
- If your primary focus is Chemical Purity: Maintain a sufficient flow rate to actively sweep away all volatile components as they are released from the skeleton.
A consistent nitrogen environment is the difference between producing high-grade carbon and producing expensive ash.
Summary Table:
| Function | Role in Carbonization | Impact on Final Product |
|---|---|---|
| Inert Atmosphere | Displaces oxygen to prevent combustion | Prevents material loss and ash formation |
| Structural Shield | Protects the developing carbon skeleton | Ensures structural integrity and density |
| Carrier Gas | Sweeps away volatile gases and tars | Increases chemical purity and prevents re-deposition |
| Flow Stability | Maintains consistent pressure and environment | Guarantees batch uniformity and high yield |
Maximize Your Carbonization Yield with KINTEK
Don't let unstable gas flows turn your valuable hydrochar into ash. KINTEK provides industrial-grade laboratory solutions backed by expert R&D and precision manufacturing. Our range of Muffle, Tube, Rotary, Vacuum, and CVD systems are engineered to maintain the rigorous inert environments required for high-purity carbon skeleton development.
Whether you need custom gas flow controls or high-temperature stability, our furnaces are fully customizable to your unique research or production needs. Contact KINTEK today to discover how our advanced thermal technology can enhance your material's structural integrity and purity.
Visual Guide
References
- Dipendu Saha, David Young. Nanoporous Carbons from Hydrothermally Treated Alga: Role in Batch and Continuous Capacitive Deionization (CDI). DOI: 10.3390/molecules30132848
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the function of an industrial tube furnace in NdFeB recycling? Unlock Efficient Rare Earth Recovery
- What makes tubular furnaces versatile and precise? Unlock Superior Thermal Processing Control
- How does the uniform thermal field provided by a vertical tube resistance furnace impact phase equilibrium experiments?
- How is a laboratory tube furnace utilized in the TG-DTA of silica-coated composite powders? Expert Analysis Guide
- What are the key takeaways regarding tubular furnaces and materials science? Unlock Precision Thermal Processing for Advanced Materials
- What are the differences between solid and split tube furnaces? Choose the Right Furnace for Your Lab
- What advantages do tube furnaces offer for research applications? Unlock Precision in Atmosphere and Temperature Control
- What safety precautions should be taken when using a High Temperature Tube Furnace? Essential Tips for Safe Operation



















